Paul Thompson's Research Publications
Tracking Tumor Growth Rates
in Patients with Malignant Gliomas
Annual Meeting of the Society for Neuro-Oncology, Scottsdale, AZ, Nov. 18-21, 1999.
Haney S, Thompson PM, Alger JR, Cloughesy TF,
Toga AW
Laboratory of Neuro Imaging, Dept. Neurology, Division of Brain Mapping,
UCLA School of Medicine, Los Angeles CA 90095, USA,
UCLA Dept. of Radiological Sciences,
and
Neuro-Oncology Program, UCLA School of Medicine
|
ABSTRACT
|
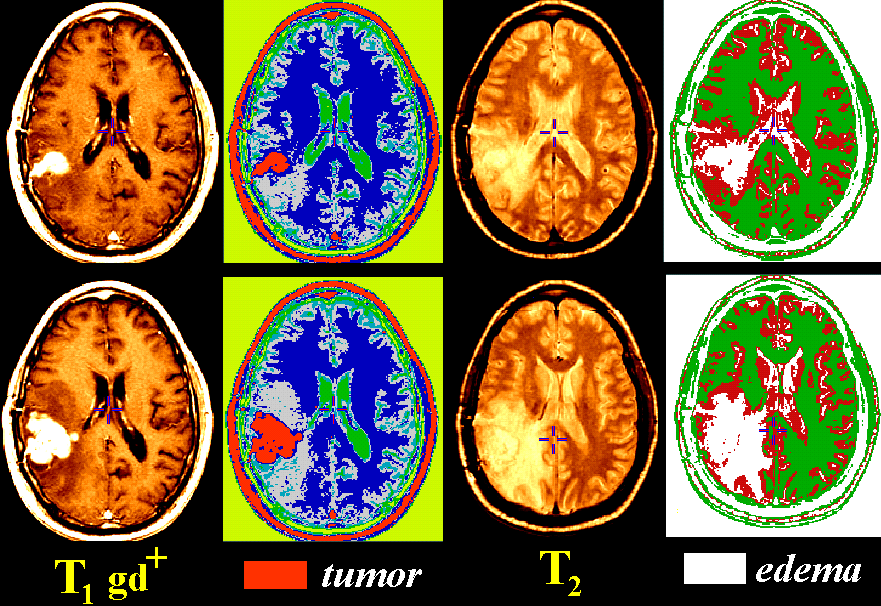
Tissue classification approaches (described below) can create maps of different tissue types
in the brain.
Together with other imaging approaches designed to assess the chemical and
genetic content of brain lesions, tissue maps can be used
to measure growth rates, determine response to therapy, and
track subtle changes in the dynamics of tumor growth.
|
Summary.
As part of a comprehensive longitudinal study of patients with
high-grade gliomas, a variety of novel pattern recognition and
3-dimensional image analysis algorithms were applied to serial MRI
scans. Rates of change were tracked in tumor, necrosis, and gadolinium-enhancing tissue
as well as
peritumoral edema, adjacent white matter, and cystic compartments,
to identify optimal algorithms and scanning protocols.
3D T2-weighted and gadolinium-enhanced T1-weighted (256x256 resolution; 3 mm
spacing) SPGR MRI volumes were acquired over a 3.5 year period (6 week to 6
month intervals) from 12 patients with glioblastoma multiforme (GBM; age:
4-54 yrs. at first scan). Scans were automatically aligned into Talairach
stereotaxic space with 6-parameter rigid transformations. Population-based
tissue maps, containing probabilistic information on tissue locations in
stereotaxic space, were automatically aligned with the scan data, adjusted
for herniation effects with non-linear registration, and used to determine a
Gaussian mixture distribution reflecting the intensities of specific tissue
classes at each time-point in the scan series. A nearest neighbor algorithm
was used to differentiate tissue types, and its accuracy confirmed by
tagging 160 tags per anatomical region (170 when a cystic compartment was present). Tissue
maps were manually adjusted to better delineate class boundaries and the
results of automated and manual segmentations were compared.
Results. T1-based segmentations provided excellent tissue differentiation
and growth rate evaluation for tumor, necrosis, cysts and enhancement.
Volumetric changes in tumor tissue ranged from -73% to +2600% corresponding to
a halving time of 130 days and a doubling time of 13.8 days. Vasogenic edema, however,
was optimally detected by classifying T2-weighted scans.
Absolute edema volumes, vital in monitoring blood-brain barrier integrity
and extravasation of plasma water, were systematically 16.5 +/- 8.8% lower
in T1-based maps (average volume: 70.5 +/- 55.9 cm3)
than in T2-based maps (average volume: 82.5 +/- 63.3 cm3; p < 0.001, paired
t-test). Nonetheless, volumetric changes detected by each type of map were
highly correlated (r2=0.98).
Conclusions. Automated tissue mapping provides significant power in tracking
the regional dynamics of multiple tissue classes in glioma patients. The resulting
tissue classification and growth rate detection systems also provide a 3D
structural framework to correlate spectroscopic, diffusion imaging, and
histopathologic indices in interventional studies. They may also be useful for
quantitative evaluation of therapeutic response in individual patients and clinically-defined
groups.
Grant Support: (to P.T. and A.W.T.): NIMH/NIDA (P20 MH/DA52176),
P41 NCRR (RR13642); (A.W.T.): NLM (LM/MH05639), NSF
(BIR 93-22434), NCRR (RR05956) and NINCDS/NIMH (NS38753).
Related Publications
(back to main list)
Contact Information
Mail:
Paul Thompson
73-360 Brain Research Institute
UCLA Medical Center
10833 Le Conte Avenue
Westwood, Los Angeles CA 90095-1761, USA.
E-mail:
thompson@loni.ucla.edu
Tel: (310)206-2101
Fax: (310)206-5518
