|
|
|
|
Paul M. Thompson, Katherine L. Narr, Rebecca E. Blanton, Arthur W. Toga
Laboratory of Neuro Imaging, Department of Neurology, Division of Brain Mapping, UCLA School of Medicine, Los Angeles, California 90095
|
|
|
|
CONTENTS
I. Challenges in Mapping the Corpus Callosum
I. Challenges in Mapping the Corpus Callosum The rapid growth in brain imaging technologies has been matched by an extraordinary increase in the number of investigations focusing on the structural and functional organization of the brain. An intense amount of research has been directed towards analyzing the structure and function of the corpus callosum, the main fiber tract connecting the two brain hemispheres, which consists of approximately 200-350 million fibers in man (Aboitiz et al., 1992a,b). Surgical transection of this structure in humans provides evidence that the corpus callosum functions to communicate perceptual, cognitive, mnemonic, learned and volitional information between the two brain hemispheres (Bogen et al., 1965). Given the importance of sensory, motor and cognitive callosal relay between hemispheres it is not surprising that this anatomic region has been a focus of studies examining structural and functional neuropathology. Given the corpus callosum's key role as the primary cortical projection system, any focal or diffuse abnormalities of bilaterally connected cortical regions may be expected to have secondary effects on homotopically distributed fibers in the callosum. These effects are observable at both cellular and gross anatomic scales (Innocenti, 1994). Effects on regional callosal structure have been reported in schizophrenia (Woodruff et al., 1995; DeQuardo et al., 1996), attention deficit hyperactivity disorder (Giedd et al., 1994; Baumgardner et al., 1996), relapsing-remitting multiple sclerosis (Pozzilli et al., 1991), Alzheimer's Disease (Hofmann et al., 1995; Vermersch et al., 1996; Janowsky et al., 1996), multi-infarct dementia (Yoshii et al., 1990), and a range of neurodevelopmental disorders and dysplasias (Sobire et al., 1995). Structural Changes. Analysis of regional neuroanatomy and callosal structure are key factors in the radiologic assessment of a wide range of neurological disorders. Nonetheless, extreme variations in brain structure make it difficult to design computerized strategies that detect and classify abnormal structural patterns. Intense controversy exists on the question of whether different callosal regions undergo selective changes in each of these disease processes. To distinguish abnormalities from normal variants, a realistically complex mathematical framework is required to encode information on anatomic variability in homogeneous populations (Grenander and Miller, 1994; Mazziotta et al., 1995). Additional controversy surrounds studies which have identified specific differences in callosal structure related to gender (DeLacoste-Utamsing and Holloway, 1982), handedness (Witelson, 1985), IQ (Strauss et al., 1994), musical ability (Schlaug et al., 1995), and in studies comparing monozygotic and dizygotic twins (Oppenheim et al., 1989). Anatomically-specific relationships between callosal structure, cortical asymmetry, and a range of cognitive measures have generated great interest, in view of the light they shed on the organization of brain function and the nature of interhemispheric communication. In this chapter, we review current neuroimaging research on callosal structure. We emphasize the many recent mathematical and methodological advances in the field. A range of imaging and computational methods are discussed for analyzing and understanding the extremely complex dynamic processes that affect callosal anatomy in the healthy and diseased brain. We also review progress in constructing a probabilistic reference system for the human brain, which can be used to analyze callosal anatomy (Mazziotta et al., 1995; Thompson et al., 1997, 1998). MRI-based image archives from large human populations are stratified into subpopulations according to age, disease state, gender and other demographic criteria, to produce population-specific representations of anatomy. The resulting brain atlases encode information on population variability, with the goal of identifying group-specific patterns of brain structure. Computerized strategies are developed to (1) detect abnormal structure in disease, with a particular focus on the corpus callosum, (2) relate detected callosal anomalies to changes in the 3-dimensional patterns of cortical asymmetry and structural variability, and (3) relate callosal changes found in development and aging to changes found in Alzheimer's Disease and schizophrenia. Growth Patterns. Later in the chapter (Section 5), we discuss methods for mapping dynamic patterns of growth at the corpus callosum. The callosum undergoes profound changes in morphology, fiber composition and myelination during brain development, and these patterns are dramatically altered in disease. In many ways, static representations of callosal structure are ill-suited to determining the dynamic effects of development and disease. Several algorithms are used to create 4-dimensional quantitative maps of complex growth patterns across the corpus callosum, based on time-series of pediatric MRI scans (Thompson et al., 1998; Thompson and Toga, 1998). Mathematical techniques are designed to analyze local growth rates, revealing the local magnitude and principal directions of dilation or contraction. Serial scanning of human subjects, coupled with computational methods for analyzing the changing callosal geometry, show promise in enabling disease and growth processes to be tracked in their full spatial and temporal complexity. II. Maps of the Corpus Callosum Neuroimaging studies of the corpus callosum are easier to understand if its elaborate internal organization
is considered. The corpus callosum connects the cortical surfaces of the two brain hemispheres, and there is a topographically
specific organization of callosal fibers in relation to the cortical regions they connect. Tract-tracing studies using
anterograde or retrograde labels such as biocytin or rhodamine-labeled latex microspheres (Innocenti, 1994) have established
the topographic distribution of callosal connections at the cortex in several species. A massive perinatal loss of callosal
axons, lasting from the 35th gestational week to the end of the first post-natal month (Clarke et al., 1989; La Mantia and
Rakic, 1990) is thought to lead to a restricted pattern of adult callosal connections (Innocenti, 1994). In the adult callosum,
the genu (or anterior third) connects pre-frontal cortices, the midbody (middle third) connects motor, somatosensory and
auditory cortices, and the splenium (posterior fifth) carries temporal, parietal, and occipital (visual) fibers.
Perisylvian fibers from superior temporal and parietal cortex relay information from critical language and association areas,
and cross mainly in the isthmus (just anterior to the splenium; see Fig. 1). To a certain degree, callosal fiber types are also
organized topographically. Fast-conducting, large diameter (>3 micrometer) sensorimotor fibers are concentrated in the posterior midbody and splenium, while thinner, more lightly myelinated fibers are found at the genu. These fibers at the genu offer a lower conduction velocity, connecting pre-frontal regions implicated in longer-term planning and organization of behavior (Aboitiz et al., 1992). Nonetheless, the idea of a sharply-defined cortical map at the callosum has been mitigated by recent anterograde tracer studies in humans (Di Virgilio and Clarke, 1997). These suggest that heterotopic connections (i.e., between non-equivalent cortical areas in each brain hemisphere) are numerous and widespread, even in the genu and splenium where callosal axons are most highly segregated. Partitioning Approaches Because there are no gross anatomical landmarks that clearly delimit anatomically or functionally distinct callosal regions, several geometric partitioning schemes have been designed to subdivide the callosum into subregions whose fiber topography is expected to be different (Fig. 1). These partitions define subregions which might be affected differently in development or disease, and whose structural parameters (such as size, shape, or MRI signal intensity) might correlate more or less strongly with cognitive test data that evaluate different channels of interhemispheric communication (Clarke and Zaidel, 1994).
Growth Patterns
Childhood and Adolescence
Childhood Disorders
Regional Topography of Callosal Growth
VII. Alzheimer's Disease and Dementia
Post Mortem and Imaging Studies
X. Conclusion
Vertical Partitions. Most studies of gender and handedness effects on callosal structure have been based on the Witelson partition (1989; Fig. 1(c)). This scheme defines callosal subdivisions based on fractions of its maximum anterior-posterior length. Nonetheless, the curvature and shape variability of the midsagittal callosum can bias the proportions of callosal area represented in each of the resulting segments. This difficulty has led several investigators to base their partitions on a curvilinear reference line (Fig. 1(d); Clarke et al., 1989) which takes the global curvature of the callosum into account.
Radial Partitions. Based on the centroid, or 'center of mass' of the corpus callosum, angular rays can be defined (Fig. 1(e)) which intersect the callosal boundary above and below. These rays can be used to produce an equiangular partition with 100 separate elements (Fig. 1(e); Rajapakse et al., 1996). Clarke et al. (1989) partition this medial reference line into nodes of equal separation, prior to defining 30 sectors based on the shortest line through each node connecting outer and inner boundaries (Fig. 1(d)). Stievenart et al. (1997) partition the callosum by defining rays normal to a series of equidistant nodes on the ventral callosal boundary (Fig. 1(g)), which provide the basis for thickness and curvature measurements. Allen et al. (1991) noted that the tip of rostrum is occasionally difficult to identify, which may add error in defining the curvilinear partitions while affecting only the rostral sector in the straight line-based approaches (Fig. 1(a),(c)).
To avoid making arbitrary definitions, Denenberg et al. (1991) performed a factor analysis to determine a 'natural' partition of the callosum. Thickness measurements were obtained from a population of 104 normal adults (by connecting 100 equally spaced points on the inner and outer callosal boundaries; Fig. 1(f)), and these measures were used to determine seven regions with consistent variations (7 factors). While the partitioning scheme chosen ultimately depends on the application objectives and the scale of the expected structural effects (Bookstein, 1996), many apparent conflicts among different callosal studies derive from hidden or overt methodological differences, as will be seen in the following sections.
III. Sex Differences in the Corpus Callosum
Intense controversy surrounds reports of regional sex differences in the anatomy of the corpus callosum (DeLacoste-Utamsing and Holloway, 1982; Clarke et al., 1989; Beaton, 1997; Bishop and Wahlsten, 1997). In 1982, DeLacoste-Utamsing and Holloway found that the area of the splenium (defined as the posterior fifth of the callosum) was larger in 5 female than in 9 male brains examined post mortem (p < 0.0895, without correction for multiple comparisons, or for brain weight), despite the fact that male brains were heavier overall, and total callosal area did not differ. In 1986, the same group reported total callosal area and maximal splenial width (SW; Fig. 1(d)) to be greater post mortem in 8 females (age: 53-87 yrs.) than in 8 males (35-81 yrs.). The result for maximal splenial width was also found post mortem in fetuses (N=32; 19 males; 11-40 weeks gestation; Holloway and DeLacoste-Utamsing, 1986).
Three subsequent studies, however (Bleier, 1985; Demeter et al., 1985; Nasrallah, 1985), reported a failure to confirm the original findings of a sex difference in adult post mortem specimens. The fetal result was also not replicated by Clarke et al., (1989) (N=32; 16 males; 20-42 weeks gestation); the same study, however, reported a larger fraction of callosal area in the posterior fifth in adult females (using a curvilinear reference line; Fig. 1(d); N=58; 32 males), although this effect was not detectable in a smaller subsample of MRI scans (N=12; 5 males). Clarke et al. also reported a larger bulbosity index in post mortem adult females (p<0.003; N=46; 27 males; one outlier excluded). The bulbosity measure was designed to provide a non-dimensional index of splenial shape, by comparing splenial and pre-splenial thicknesses. Defined as (Tmax-Tmin)/Tmin, this measure is based on the maximal (Tmax) and minimal (Tmin) pre-splenial thicknesses found among the posterior 10 thickness measurements. In this scheme, thickness values were measured in a 30-segment radial partition (Fig. 1(d)) using the shortest line connecting outer and inner boundaries which also passed through equally-spaced nodes on the curvilinear reference line.
Age x Sex Interaction Effects. Witelson (1989; 1991) detected an age-related decrease in total callosal size (without correction for overall brain size) in male but not female post mortem specimens (N=62; 23 males aged 26-69 yrs., mean age 54; 39 females aged 35-65 yrs., mean age 52). Similarly, Burke and Yeo (1994) found a greater age-related decline in callosal area in males (N=97 MRI scans; all subjects right-handed; 38 males). If gender-related changes in callosal size with age are confirmed (i.e., if an age x sex interaction occurs; cf. Byne et al., 1988), comparisons between the sexes at even slightly different ages could be prone to misinterpretation. Some callosal studies have been criticized for comparing subject groups with large age variations (Beaton, 1997). Age-related effects may be exacerbated in developmental and embryonic studies, where gestational or chronological age may not be a reliable index of developmental state. In pediatric studies, significant age-related changes may occur over periods of weeks or months (Allen et al., 1991; Thompson et al., 1998).
In view of possible age-related effects, Allen et al. (1991) performed a large-scale age-matched MRI study (N=122; 61 males aged 16-78, mean 42.1 yrs., 61 females aged 16-79, mean 42.9 yrs.). In this study, the maximum width of the splenium (SW; Fig. 1(d)) was found to be significantly greater in adult females (without adjustment for head size), as was a different measure of bulbosity, namely the percentage by which the average width of the splenium (defined, again, as the posterior fifth in a curvilinear partition) exceeded the average width of the adjacent sectors. A 23.2% greater value was found in females when the bulbosity coefficient measured how much splenial widths exceeded the average width of the rest of the posterior half. No individual sector area was sexually dimorphic (p>0.05 after Bonferroni correction), irrespective of whether areas were expressed as a proportion of total callosal area, and whether or not areas were defined using a straight-line or curvilinear partition (Fig. 1).
Brain Size Effects. Additional controversy surrounds the practice of expressing measures of specific brain structures as a proportion of overall brain size, which is known to be significantly different between the sexes. The mean volume of the forebrain, where all callosal fibers originate, is about 9% higher in adult young men than in age-matched women (men: 1.08± 0.11 liters (N=71, age: 25.3± 4.6), women: 0.99± 0.10 liters (N=49, age:26.3± 4.9); Jäncke et al., 1997). A similar 10% sex difference in mean post mortem brain weight has also been reported by Pakkenberg and Voigt (1964).
In considering size differences for specific brain structures, it is important to know whether a group difference can be explained by a mere difference in overall brain volume, or whether it reflects the influence of additional factors (sex, handedness, disease conditions) independent of brain volume effects. In an attempt to factor out the effects of gross variations in brain size, early studies of the callosum used the ratio of total callosal area to a measure of whole brain (or forebrain) size. Weight measures were typically used in post mortem studies, while volume or midsagittal cerebral area were used as correction factors in neuroimaging studies. Although these corrections are performed because total callosal area is correlated with brain weight (r=0.29, Bishop and Wahlsten, 1997), it was found that, despite this correction, the callosal area/brain weight ratio is still highly correlated with brain weight even though brain weight has supposedly been divided out (r=-0.314; N=95; Bishop and Wahlsten, 1997). This indicates that ratio measures do not fully adjust for brain size. To assess the effects of various factors on regional callosal measures, brain size measures should instead be added as a covariate in ANCOVA or multiple correlation type analyses. Unfortunately, even these linear statistical models expect callosal measures to vary linearly with extraneous parameters such as brain size. Ultimately, a power law may be more appropriate, as will be seen in the next sections.
Jäncke et al. (1997) suggested a log-linear relation exists in adults between total callosal area (CC) and forebrain volume (FBV), with CC = constant x (FBV)p. Estimates of the power p (0.66 in females, 0.52 in males) were based on significant regressions in an MRI cohort of 120 subjects. Since callosal area increases less than linearly with forebrain volume (i.e. p < 1), and since women have smaller brains on average, the ratio of total callosal size to brain volume will automatically be larger in women in the absence of other factors. This indicates a general brain size effect, which takes place independent of gender, so that the altered proportion is not a specialized feature of the structure being analyzed (Clarke et al., 1989; Jäncke and Steinmetz, 1997).
As a brain size correction, the pth power of FBV, estimated empirically from the sample, is likely to provide a useful covariate for multivariate statistical tests, if removal of brain size effects is required. However, an even more general model may be required if the value of the exponent p were also found to depend on gender. In addition, the ratio of callosal area to an area-based measure of brain size, namely the sum of cerebral areas in an axial and a sagittal cut (Rauch and Jinkins, 1994), has been found to rise rapidly with age from birth to 20 years. This ratio rose from 1% to 4% across this 20 year span (although no gender differences were detected in the overall rate of increase; Rauch and Jinkins, 1994). As a result, sex differences in any proportion measures (such as 'splenial area as a proportion of total callosal area') must be interpreted with caution, since any association between sex or age and the ratio's denominator can create a substantial effect. As a result, significant sex differences in ratio measures have often been found to disappear when analysis of covariance is used on the same data (Bishop and Wahlsten, 1997).
Measurement using Deformable Brain Atlases. Engineering approaches based on deformable templates (Fig. 1(h)) have also been applied to detect differences in callosal shape due to sex, disease or growth processes (Davatzikos et al., 1996; Bookstein et al., 1997; Thompson et al., 1997, 1998). The basic idea of these approaches is outlined in the next few sections.
Digital brain atlases are templates which represent labeled anatomical structures from a typical subject or specimen in a 3-dimensional coordinate system. Design of a brain atlas to represent human populations is complicated by drastic cross-subject variations in anatomy, and a fixed atlas based on a single subject may fail to represent the anatomy of new subjects. Typical solutions to this problem involve deformable, probabilistic, or hybrid atlases, which expand the atlas concept to represent large human populations (Mazziotta et al., 1995; Thompson et al., 1997; Thompson and Toga, 1998; for a review, see Toga and Thompson, 1998).
Deformable atlases are brain atlases which can be adapted to reflect the anatomy of new subjects (Evans et al., 1991; Gee et al., 1993; Christensen et al., 1993; Sandor and Leahy, 1995; Rizzo et al., 1995; Haller et al., 1997). Image warping algorithms, specially designed to handle 3D neuroanatomic data, apply complex profiles of dilation and contraction to a digital brain atlas, reconfiguring it into the shape of the patient's anatomy (see Fig. 2).

|
|
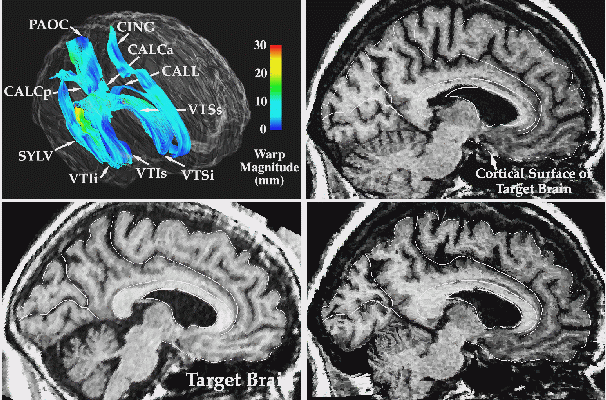
Fig. 2. A Deformable Brain Atlas: Mapping a 3D Digital Cryosection Volume onto 3D MRI Volumes. The result of warping a 3D cryosection brain atlas (top left) into the shape of a target MRI anatomy of (top right) is shown (lower left), with callosal and cortical landmarks of the target anatomy superimposed. Note the reconfiguration of the callosum, as well as major occipital lobe sulci, into the shape of the target anatomy. This type of registration of callosal, sulcal and cytoarchitectural boundaries can be used to measure neuroanatomic differences in individual subjects or groups (see Section 3; Figs. 5-10). 3D mapping of very local differences in structure (lower right) is possible only with a high-dimensional warping technique (Thompson and Toga, 1996; Davatzikos et al., 1996; Christensen et al., 1996). |
Sex Differences. To examine sex differences at the callosum, Davatzikos et al. (1996) derived a digital template of the callosum from the Talairach brain atlas (Talairach and Tournoux, 1988). This template was then deformed, using image warping, to match individual callosal outlines from 8 male and 8 female subjects' MRI scans. Analysis of the required deformation fields showed that, in some areas, the average dilation for the female group was greater than the average dilation for the male group. Dilation values were defined mathematically as the local Jacobian determinant, or local 'expansion factor', of the deformation field (see Fig. 1(h)), and these values were normalized with respect to total callosal area. The sex difference was most prominent in a vertical strip which crossed the splenial region. Evidence for a local sex difference was expressed as an effect size, indicating that the sex difference in mean dilation values exceeded the overall standard deviation of the dilation values in parts of the splenium (Davatzikos et al., 1996).
Shape-Theoretic Approaches. The data acquired by Davatzikos et al. (1996) was subsequently
re-analyzed by Bookstein (1997) using shape-theoretic methods. In 'Procrustes' methods, developed for the statistical
analysis of biological shape (Bookstein, 1989; 1997), a series of points are spread out along the callosal boundary,
and used to derive a warping field which matches one boundary with another. Affine components of neuroanatomic difference
are first factored out by rotating and scaling configurations of point landmarks in each subject into least-squares
correspondence with a Procrustes mean shape. Residual deformations which reflect individual anatomic differences are
then expressed in terms of an orthogonal system of principal deformations derived from the bending energy matrix of
the differential operator which governs the deformation (Bookstein, 1997). The deformations produced by image warping
algorithms which match biological shapes are often governed by a differential operator which controls the way in which
one anatomy is deformed into the other (for a technical review, see Thompson and Toga, 1998).
Examples include the Cauchy-Navier operator of linear elasticity ![]() ,
used by Davatzikos et al. (1996), and by Thompson and Toga (1998), and the biharmonic, or thin-plate spline
operator, used by Bookstein (1989, 1997).
The properties of the governing operator can be used to make the deformation reflect the mechanical properties of deformable elastic or fluid media.
,
used by Davatzikos et al. (1996), and by Thompson and Toga (1998), and the biharmonic, or thin-plate spline
operator, used by Bookstein (1989, 1997).
The properties of the governing operator can be used to make the deformation reflect the mechanical properties of deformable elastic or fluid media.
In Bookstein's approach (1989), a number of modes of variation, based on the eigenvectors of the covariance matrix of landmark positions, can be determined to describe the main factors by which the instance shapes tend to deform from the generic shape. Of particular relevance are methods used to define a mean shape, and distances between shapes (Procrustes distances), in such a way that conventional statistical procedures can be applied to characterize shape differences between two groups. In his re-analysis of the callosal data acquired by Davatzikos et al. (1996), Bookstein (1997) used a permutation test to determine whether the Procrustes shape distance between the mean callosal shapes was significantly greater when the outlines were split into male and female groups than when groupings were randomly assigned. As indicated by the effect size analysis of Davatzikos et al. (1996), this shape-theoretic analysis identified a shape difference between the sexes in the posterior half of the callosal boundary (p ~ 0.03). These approaches are also being applied to detect callosal shape anomalies in schizophrenia (DeQuardo et al., 1996; Bookstein, 1997).
Orthogonal Functions and Fourier Series Approaches. Shape-theoretic approaches, described above, express shape differences between anatomical boundaries in terms of statistically orthogonal functions, namely principal components of covariations of position (Bookstein, 1997). Geometrically orthogonal functions, such as elliptical Fourier series (Staib and Duncan, 1992), Oboukhoff expansions (Joshi et al., 1998), and spherical harmonics (Thompson and Toga, 1996; Joshi et al., 1998) have also been used to represent anatomical shapes. These representations provide a frequency-based decomposition of an object and describe its overall shape efficiently using a few parameters, which can be analyzed statistically. Due to their representation efficiency, and ability to represent a large class of shapes, elliptical Fourier series have also been used in automated methods to find the callosal boundary in MRI images (Staib and Duncan, 1992). This contour detection method operates by tuning the Fourier coefficients of an estimated callosal shape, until a goodness-of-fit measure is optimized. Elliptical Fourier series also provide a means to generate average callosal shapes for specific subject groups, and they have been used to quantify large-scale changes in callosal anatomy during brain development (Ferrario et al., 1996).
Local Shape Operators. In view of the large number of harmonics required to represent complex shapes, descriptors based on orthogonal functions are less suitable for detecting local structural differences. Small scale shape differences, such as callosal differences which may exist due to sex or handedness, are likely to be detected optimally using local shape operators such as the delta-filter (Bookstein, 1997), and other tensorial operators which act on deformation fields to quantify local shape differences (Thompson and Toga, 1998; see also Thirion and Calmon, 1997; Thirion et al., 1998). These operators are used to detect growth patterns at the callosum in Section 5, and to detect multiple sclerosis lesion growth in (Thirion and Calmon, 1997). The statistical significance of these local deformation maps can be assessed by modeling the dilation maps as a log-normal distributed random scalar field (cf. Thirion et al., 1998; Ashburner et al., 1997), or by estimating the parameters of a Gaussian random vector field as a means to assess shape differences between groups (see Section 9; Thompson and Toga, 1997, 1998; Cao and Worsley, 1998). Nonetheless, it seems that any method, however powerful, must be combined with large sample sizes, and carefully controlled for extraneous demographic factors such as age, head size, and handedness. Only then is the controversy over sex differences in callosal anatomy likely to be resolved.
IV. Development of the Corpus Callosum
Embryonic Period
Growth of the normal human brain occurs in a highly regular and well-defined manner. Anatomic neuronal development progresses in a posterior to anterior as well as ventral to dorsal fashion. Growth of the corpus callosum also occurs in an orderly manner. The development of the corpus callosum is initiated between 8-17 weeks of gestation (Rakic and Yakovlev, 1968). A thickening of the telencephalon, along the rostral wall, forms the lamina reuniens, which is the precursor to the white matter bundles of the anterior commissure and corpus callosum. As cells from the lamina reuniens migrate superiorly they form the massa which will form the bed for the extension of the crossing fibers of the corpus callosum (Barkovich and Norman, 1988). The corpus callosum does not develop homogenously, and axons of the genu develop first followed by the body and splenium. One exception to this anterior to posterior growth pattern is the rostrum. This is the last component of the callosum to project crossing fibers, at approximately 18-20 weeks gestation (Rakic and Yakovlev, 1968).
The major components of the corpus callosum are established prenatally, yet development is far from complete at birth. Although neuronal differentiation has concluded at birth, myelination of cortical axons has just begun. Myelination is the process whereby axons become encased by myelin sheaths. This process insulates axons and enhances the speed of neuronal conduction (Yakovlev and Lecours, 1967). Myelination of the central nervous system generally occurs in a caudal to rostral fashion. Spinal cord and brain stem pathways myelinate first, during prenatal stages, and frontal and association areas of the cerebrum myelinate postnatally (Barkovich and Kjos, 1988). Continued myelination has been reported as late as the third decade of life, and this may reflect increased efficiency in the synthesis of information (Yakovlev and Lecours, 1967).
Parallelling the growth processes in the cerebrum, myelination of callosal axons also proceeds in a posterior to anterior fashion and may be the primary component of the growth observed at the callosum (Georgy et al., 1993). Rakic and Yakovlev (1968) report that the corpus callosum more than doubles in size between birth and two years of age. Barkovich and Kjos (1988) also observed that the newborn corpus callosum appears thin and flat, with substantial thickening occurring around 3 months of age. Substantial area increases are seen in the splenium, followed by more gradual increases in the body and rostrum (Kier and Truwit, 1996). As noted earlier, the genu of the corpus callosum consists mainly of fibers which traverse inferior frontal and anterior/inferior parietal regions, while the splenium carries fibers from homologous visual and visual association areas (DeLacoste et al., 1985). The observed pattern of callosal development is therefore not surprising: sensorimotor and visual areas may be most important as the neonate develops binocular vision and becomes coordinated in various body movements such as grasping objects (Von Hoftsten, 1984; Barkovich and Kjos, 1988). Prefrontal and posterior association areas may initially be less important to the infant, as these regions integrate sensory experiences and subserve higher cognitive processes such as planning (Diamond, 1990). Increases in corpus callosum size may reflect increases in the complexity of interactions between an infant and its environment.
Childhood and Adolescence
The most dramatic developmental changes of the corpus callosum occur during the first years of life, yet continued maturational changes have been reported until late childhood and adolescence (Pujol et al., 1992; Giedd et al., 1996). Pujol et al. (1992) measured total callosal area in a cohort of 90 subjects, and found area increases until the third decade of life. In addition, cross-sectional studies of total callosal area have corroborated increases in callosal volume until adulthood (Schaefer et al., 1990; Allen et al., 1991; Rauch and Jinkins, 1994). More regional assessments of callosal development have been obtained in a group of children ranging in age from 4-18 (Rajapakse et al., 1996; Giedd et al., 1996). Developmental increases were found primarily in the area of the splenium and isthmus regions. No corresponding age effects were detected in the genu, rostrum and body. Morphological changes of the corpus callosum have also been observed with increasing age (Ferrario et al., 1996). Dramatic maturational changes were seen, as the callosum transformed from thin and flat in infants, with no bulbous enlargements, to a thicker, rounder genu and splenium in teens. As in the adult studies (Section 3), measurements of sexual dimorphism in the corpus callosum of children and adolescents have produced conflicting results. No significant sex differences were found in splenial area or shape in a post mortem sample of children ranging in age from birth to 14 years (Bell and Variend, 1985). In contrast, gender differences in callosal shape as well as total callosal area have been reported in infants as young as newborn to 14 months of age (Clarke et al., 1989).
Several authors have postulated reasons for such patterns of growth in the brain from birth to late adolescence. Neuronal cell division and migration has been reported as complete prior to birth (Carlson et al., 1988). Postnatal anatomic reorganization may arise primarily from processes such as naturally occurring cell death, synaptic pruning and myelination. Huttenlocher (1990) proposed that brain reorganization occurs into late childhood and adolescence due to synaptic refinement of functional pathways. At birth, diffuse projections have been observed in callosal connections across visual, auditory, and somatosensory cortex in young primates, kittens and rodents (Ivy and Killackey, 1981; Feng and Brugge, 1983; Innocenti, 1994). Transient callosal projections between areas have also been reported, and may suggest excess cortical connectivity during early childhood (Carlson et al., 1988).
With age, diffuse and transient axonal projections are selectively retracted, a process termed synaptic refinement. Selective elimination of axons originating from cortical locations has been observed in both cats and monkeys, where more than half of the axons produced are eliminated from the callosum during development. Furthermore, synaptic refinement has been observed in human frontal and visual cortex (Huttenlocher, 1990). Functional consequences of such refinements have been studied using PET (Chugani et al., 1987). Cerebral blood flow measurements decreased and became less diffuse with age, suggesting that less neural activity is required with increasing maturation of cognitive skills. While significant reductions in axonal projections have been reported in the corpus callosum, increases in total area have been reported. Callosal connections which are not eliminated may be strengthened by increases in axonal diameter and myelin deposition (Carlson et al., 1988). As a consequence, the speed and efficiency of the inter-hemispheric transfer of information may be enhanced. Brown and Jaffe (1975) suggest that the two hemispheres may not be completely specialized at birth. Refinement of axonal connections and increases in axonal size may reflect continued development in functional specialization of the cerebral hemispheres. Thus, growth of the callosum into late adolescence and early adulthood may reflect a balance between synaptic refinement and increases in myelination and diameter of axonal projections. These processes may ultimately serve to increase functional specialization of the hemispheres, allowing acquisition of more agile and finely coordinated skills.
Childhood Disorders
Attention Deficit Hyperactivity Disorder (ADHD). ADHD has been characterized as a disorder in attentional and motor systems, and is thought to arise from deficits in frontal lobe circuitry. The corpus callosum has been studied in ADHD to assess possible abnormalities in commissural circuits. Decreases in the area of the splenium (Semrud-Clikeman et al., 1994; Lyoo et al., 1996), genu (Hynd et al., 1991) and in the rostrum and rostral body have all been reported (Giedd et al., 1994; Baumgardner et al., 1996). Findings of reductions in anterior callosal regions (genu, rostrum, and rostral body) suggest abnormalities of information transfer in prefrontal, premotor, and anterior cingulate regions (Witelson et al., 1989). These callosal deficits coincide well with data suggesting that anterior cingulate and prefrontal regions are involved in attentional regulation (Steere and Arnsten, 1995). However, posterior association areas have been suggested to play a role in sustained attention, and deficits here may explain abnormalities found in posterior regions of the callosum (Semrud-Clikeman et al., 1994).
Autism. Social and cognitive abnormalities suggest that autism may be a disorder of information processing (Saitoh et al., 1995). Autistic children fail to make eye contact, and rarely use language to communicate (Cole and Cole, 1993). Structural abnormalities have included regional brain enlargement in posterior, temporal, and occipital but not frontal cortices (Piven et al., 1997). The corpus callosum has been examined to assess a possible excess cortical connectivity resulting from such enlargements. The splenium of the corpus callosum was reported as smaller in autistic patients as compared to controls, with a trend for reductions in isthmus/posterior body regions (Saitoh et al., 1995; Piven et al., 1997). Yet, a lack of differences in corpus callosum volume between autistic patients and normal controls has also been cited (Gaffney et al., 1987). The discrepancy between regional enlargement of brain hemispheres and decreases in posterior regions of the corpus callosum may suggest increases in ipsilateral connections, with concomitant decreases in contralateral callosal connections. This suggests that autism may be a disorder arising from detrimental increases in ipsilateral connections.
Down's Syndrome. Down's syndrome is the most frequent genetic cause of mental retardation, and is a chromosomal disorder with patients having one more chromosome than normal (Cole and Cole, 1993). Corpus callosum abnormalities have been reported, with associated decrements in anterior regions (Wang et al., 1992). These results are not surprising, because Down's syndrome patients also have reductions in frontal lobe volume (Jernigan et al., 1993). Furthermore, it has been reported that subjects with Down's syndrome display poor verbal fluency and impaired performance in problem-solving strategies (Wang et al., 1992). Thus, in the case of Down's syndrome, interhemispheric transfer of semantic information may be compromised by deficits in frontal lobe and anterior callosal regions.
Dyslexia. Dyslexia has been characterized as a disability in reading performance observed in conjunction with average or above average intelligence (Hynd et al., 1995). More specifically, it has been suggested that some dyslexics have deficits in the phonological processing of information (Rumsey et al., 1992). Studies of callosal morphology in dyslexia have reported a smaller callosal genu (Hynd et al., 1995), as well as larger splenium and total callosal area (Duara et al., 1991; Njiokiktjien et al., 1994). Furthermore, Hynd et al. (1995) found correlations between reading achievement and the relative size of the splenium and genu in dyslexic patients. Abnormalities of the corpus callosum in dyslexic patients may imply a role for the corpus callosum in the interhemispheric transfer of phonological information. Evidence for this comes from findings that callosal agenesis patients are impaired in phonological as compared to lexical reading (Temple et al., 1990).
Fetal Alcohol Syndrome. Fetal exposure to alcohol can have several morphological effects, such as CNS and facial abnormalities, depending on the timing of maternal exposure to alcohol and the quantity consumed (Johnson et al., 1996). Several abnormalities of the corpus callosum have been reported in this disorder (Riley et al., 1995; Johnson et al., 1996; Swayze et al., 1997). Abnormalities include complete or partial agenesis, thinning of the posterior body, and area decreases in rostral regions. Deficits of the corpus callosum may arise from excessive or premature cell death, as well as an inhibition of axons which cross the midline (Swayze et al., 1997). Johnson et al. (1996) hypothesize that the teratogenic effects of alcohol may suppress neuronal activity, and thus impair activity-dependent neuronal development. Results such as these suggest that callosal development may be an activity-dependent process, which is particularly susceptible to the harmful effects of alcohol.
Tourette's Syndrome. Tourette's syndrome is characterized by vocal and motor tics, and has commonly been referred to as a disorder involving the basal ganglia (Hyde et al., 1995). Total corpus callosum volume has been examined in adult patients and was found to be significantly reduced (Peterson et al., 1994) On the other hand, increases in the size of the rostrum have been reported in child and adolescent populations, even after correcting for intracranial volume (Baumgardner et al., 1996). It was suggested that a larger corpus callosum in childhood may cause an overcompensation in synaptic refinement, and result in a smaller corpus callosum in adulthood. Baumgardner et al. (1996) further propose that a callosal enlargement in childhood may amplify inter-hemispheric transfer of information, thus decreasing control of a behavior specialized to a particular hemisphere.
V. Mapping Dynamic Growth Patterns during Development
Temporal Maps of Brain Structure. Current structural brain imaging investigations focus on the analysis of 3-dimensional models of brain structure, derived from volumetric images acquired at a single time-point from each subject in the study. In many ways, static representations of structure are ill-suited to determining the dynamic effects of brain development or disease. However, serial scanning of human subjects, when combined with a powerful set of warping and analysis algorithms, can enable disease and growth processes to be tracked in their full spatial and temporal complexity.
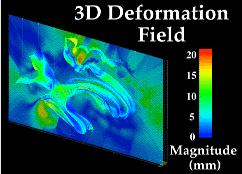
Three-dimensional image warping algorithms, specially designed to handle neuroanatomic data, provide a means to compute extremely complex maps of anatomical differences between different subjects (Fig. 2,3; Thompson and Toga, 1996).
|
Fig. 3. 3D Image Warping Measures Patterns of Anatomic Differences. T1-weighted MR sagittal brain slice images from (left, (a)) a normal elderly subject’s scan, (b) a 'target' anatomy, from a patient with clinically-determined Alzheimer's disease; and (c) result of warping the reference anatomy into structural correspondence with the target. Note the precise non-linear registration of the callosal and cortical boundaries, the desired reconfiguration of the major sulci, and the contraction of the ventricular space and cerebellum. The complexity of the recovered deformation field is shown by applying the two in-slice components of the 3D volumetric transformation to a regular grid in the reference coordinate system. This visualization technique (d) highlights the complexity of the warping field in the posterior frontal and cingulate areas, corresponding to subtle local variations in anatomy between the two subjects. To monitor the smooth transition to the surrounding anatomy of the deformation fields initially defined on the surface systems, the magnitude of the warping field is visualized (e) on models of the surface anatomy of the target brain, as well as on an orthogonal plane slicing through many of these surfaces at the same level as the anatomic sections. Note the smooth continuation of the warping field from the complex anatomic surfaces into the surrounding brain architecture, and the highlighting of the severe deformations in the pre-marginal cortex, ventricular and cerebellar areas.
Warping Algorithms. Any comprehensive framework for modeling temporal change in 3-dimensional brain structure must draw upon methods for quantitating its material transformation between pairs of images acquired at successive time-points. Warping algorithms are central to all of these approaches. The warp is a 4D model. It specifies the displacement of every anatomic point in the brain across the disease or developmental stage spanned by the two images. As such, it permits complete morphometric quantitation of the dynamic effects of the underlying biological processes on the geometry of the brain and its substructures. It allows points, surfaces and curved anatomic interfaces to be matched up in a pair of image sets. As a result, changes in volumes, surface areas, orientations, distances and in metrical relations between substructures - as well as measures of dilation rates, contraction rates, and rates of shearing and divergence of the cellular architecture - may be computed locally, for all structures, directly from the warping field. Since the warping field assigns a displacement for every anatomic point across a time-step, curves, surfaces and volumes in an early image may be re-identified in a later one. This enables relative areas, lengths and volumes to be compared over time. Derivatives of these quantities with respect to time allow growth rates to be quantified locally for any structure; spatial derivatives of the warping field allow shearing and dilation to be measured locally, and compared for different substructures.
Mapping Growth Patterns in 4 Dimensions. In our initial human studies (Thompson et al., 1998; Thompson and Toga, 1998), we developed several algorithms to create 4-dimensional quantitative maps of growth patterns in the developing human brain, based on time-series of high-resolution pediatric MRI scans. Deformation processes recovered by the warping algorithm were analyzed using vector field operators to produce a variety of tensor maps. These maps were designed to reflect the magnitude and principal directions of dilation or contraction, the rate of strain, and the local curl, divergence and gradient of flow fields representing the growth processes recovered by the transformation.
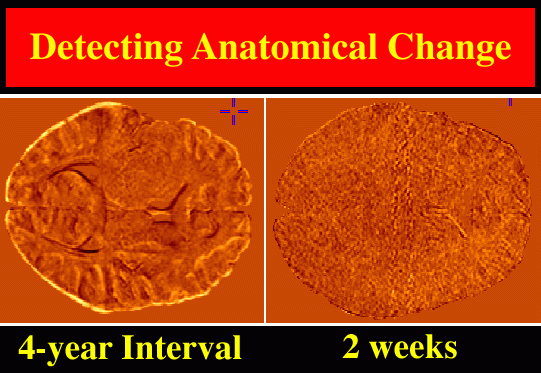
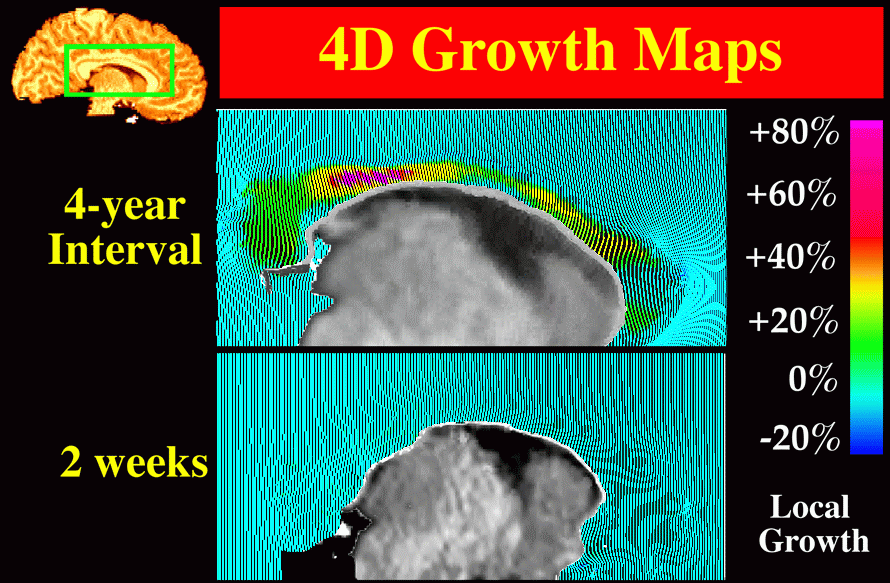
3D (2562 x 124 resolution) T1-weighted fast SPGR (spoiled GRASS) MRI volumes were acquired from young normal subjects (mean age: 8.6±3.1 yrs.) at intervals ranging from 2 weeks to 4 years. Pairs of scans were selected to determine patterns of structural change across the interval between the two scans. These scan pairs were pre-processed, with a radio-frequency bias field correction algorithm, and rigidly registered using automated image registration software (Woods et al., 1993). Registered scans were then histogram-matched and a preliminary map of differences in MR signal intensities between the two scans was constructed (Fig. 4).
|
| Fig. 4. Growth Patterns in the Developing Human Brain: Preliminary Maps of MRI Signal Differences. A young normal subject was scanned at the age of 7, and again four years later, aged 11, with the same protocol. Scan pairs were histogram-matched, rigidly registered, and a voxel-by-voxel map of intensity differences (left) reveals global growth. In a control experiment, identical procedures were applied to two scans from a 7 year old subject acquired just two weeks apart, to detect possible artifactual change due to mechanical effects, and due to tissue hydration or CSF pressure differences in the young subject between the scans. These artifacts were minimal, as shown by the difference image (right), which, as expected, is largely noise. Rigid registration of the scans is a precursor to more complex tensor models of structural change, which not only map local patterns of differences or change in 3 dimensions, but also allow calculations of rates of dilation, contraction, shearing, and torsion. |
|
|
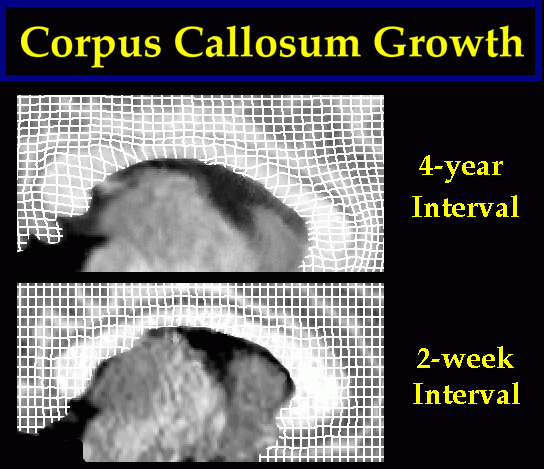
Fig. 5. Tensor Maps of Callosal Growth. (top panel:) A complex pattern of growth is detected in the corpus callosum of a young normal subject. This map illustrates structural change occurring in the 4-year period from 7 to 11 years of age. The effects of the transformation are shown on a regular grid ruled over the reference anatomy and passively carried along in the transformation which matches it with the target. Despite minimal changes in overall cerebral volume, callosal growth is dramatic, with peak values occurring at the isthmus. The pattern of growth contrasts with the near-zero maps of change observed between scans acquired over a 2-week interval (lower panel). Fig. 6. Local Analysis of Callosal Growth Patterns using Vector Field Operators. Vector field operators help to emphasize patterns of contractions and dilations, emphasizing their regional character. Here, the color code shows values of the local Jacobian of the warping field, which indicates local volume loss or gain. Pronounced neuroanatomical growth is observed during the 4-year interval (top panel). This contrasts sharply with the negligible change detected over a 2-week time-span (lower panel). |
|
Tensor Maps of Growth. In contrast to the near-zero maps of change recovered at short time intervals (2 weeks), tensor maps of growth spanning large time intervals (4 years) showed complex and heterogeneous patterns of change. In one subject scanned at ages 7, 9 and 11, comparative stability of lobar and thalamic anatomy, and negligible changes at the cortex, were accompanied by pronounced focal growth of the callosal isthmus (Fig. 5), ventricular enlargement and loss of caudate tissue.
Growth in Callosal Anatomy. To further characterize the growth process at the callosum (Fig. 5), derived properties of the deformation fields were examined (Fig. 6), including local expansion, contraction or shearing effects recovered by the warping transformation. As noted earlier (Section 3), the Jacobian of the deformation field has been used as a local index of gender-specific shape differences in the corpus callosum (Davatzikos, 1996), while here it is used to indicate its growth. Other local vector field operators, including the gradient and divergence (Thompson et al., 1998) and the specialized norm x divergence operator (Thirion and Calmon, 1997) can be applied to deformation fields. These operators are specifically designed to reveal and emphasize different aspects of growth or pathologic processes, including their local magnitude and directional biases.
Regional Topography of Callosal Growth. Fig. 6 shows the complex patterns of growth detected in a young normal subject during the 4-year period from 7 to 11 years of age. Despite minimal changes in overall cerebral volume, striking regional growth is detected throughout the callosum, with peak values occurring at the isthmus (the same area found to degenerate preferentially in our dementia studies (see Section 7; Thompson et al., 1998)). This pronounced pattern of growth contrasts with the near-zero maps of change observed over a 2-week interval (Fig. 6; lower panel).
Warping algorithms hold tremendous promise in representing, analyzing and understanding the extremely complex dynamic processes that affect regional anatomy in the healthy and diseased brain. Only with a 4-dimensional approach will patterns of callosal growth or degeneration be recorded in their full spatial and temporal complexity.
VI. Aging and the Corpus Callosum
The corpus callosum has been reported to maintain its shape and size during the third and fourth decades of life with a gradual decline in size during later years (Pujol et al., 1992). Ratio measurements of the corpus callosum as a proportion of midline internal skull surface area have been used to study aging effects, while correcting for variations in head size (Laissy et al., 1993). Significant decreases were found in total callosal area without associated reductions in total brain size in subjects ranging in age from 12-74 years. Regional aging effects on callosal morphology are most prominent in anterior regions (genu, rostrum and anterior body) while posterior regions have been found to remain stable with age (Weis et al., 1993; Parashos et al., 1993; Salat et al., 1997). However, age-related decreases have also been reported for midbody and posterior regions (Beigon et al., 1994). In addition, decreases in callosal width with age have been reported (Byne et al., 1988).
Fiber composition of the callosum has been studied to assess possible aging effects (Aboitiz et al., 1996). Age-related increases were found in the number of large-diameter myelinated callosal fibers, but no age-related increases in small diameter fibers were seen. These results may suggest increases in myelin deposition during the later years. It was implied that increases in myelin deposition may serve as a compensatory mechanism for age related decrements in efficiency of interhemispheric transfer.
Callosal T1 relaxation times, which describe the tissue-dependent decay of signals in MRI experiments, have been analyzed in an adult and elderly population (Doraiswamy et al., 1991). Positive correlations between age and callosal T1 relaxation times were found. Furthermore, a negative relationship between age and callosal area was seen. T1 relaxation values are sensitive to the environment of water in most tissues, and therefore longer callosal T1 relaxation times are suggestive of an increase in callosal hydrogen content.
Decreases in callosal size with age could have several functional consequences. One consequence may be a decline in the speed of interhemispheric information transfer. Since normal aging is associated with selective neurocognitive deficits, one possibility is that a decrease in callosal axon number can lead to a decline in performance, in various cognitive tasks. Support for this theory comes from studies which have associated callosal atrophy with impaired information processing and delayed transfer of visual information (Roa et al., 1989).
Regional age effects in anterior callosal regions correspond well with aging effects seen in the cerebrum. Axons in the anterior callosum have been reported to connect homologous regions of the frontal lobe. Previous studies have suggested that frontal lobes may be disproportionately affected by age-related changes such as sulcal widening (Tomlinson et al., 1968), volume reductions (Coffey et al., 1992), and neuronal cellular alterations (Terry et al., 1987). These results are consistent with theories suggesting that phylogenetically newer regions of the brain may be more vulnerable to aging effects than visual and sensorimotor regions (Armstrong, 1990). In addition, functional imaging studies of aging show preferential decreases in blood flow in prefrontal regions, with little changes in occipital and temporal cortex (Waldemar, 1995).
VII. Alzheimer's Disease and Dementia
Alzheimer's Disease (AD) is accompanied by a complex and distributed pattern of neuroanatomic change, which is difficult to distinguish clinically from dynamic alterations in normal aging. Diagnosis of AD prior to death remains one of exclusion. Definitive diagnosis requires post mortem histologic findings of diffuse neuronal and synaptic loss, accompanied by characteristic neuropathologic lesions (McKhann et al., 1984; Khachaturian et al., 1985) such as beta-amyloid plaques (Delaère et al., 1989), neurofibrillary tangles (Wilcock and Esiri, 1982), Hirano bodies (Katzman, 1986) and granulovacuolar degeneration (Di Patre, 1990).
Although definitive diagnosis of AD requires direct observation of autopsy or biopsy specimens with characteristic neuropathologic lesions, the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS-ARDRA) has defined criteria for probable Alzheimer’s Disease (McKhann et al., 1984). These criteria include an acquired persistent decline involving at least 3 of the following cognitive domains: language, memory, visuospatial skills, cognition, emotion or personality. The accuracy of these criteria has been evaluated at autopsy and has been demonstrated to be 80-85% (Blacker et al., 1994).
Neuroimaging in Dementia. Structural neuroimaging is increasingly important in evaluating patients with probable Alzheimer's Disease (Davis et al., 1994). Computed tomography (CT) and magnetic resonance imaging (MRI) studies in AD reveal gross cerebral atrophy, starting temporal and parietal areas (Kido et al., 1989; Erkinjuntti et al., 1993; Killiany et al., 1993). As the disease progresses, atrophy of the caudate, lenticular and thalamic nuclei are observed (Jernigan et al., 1991), together with sporadic signs of sulcal and ventricular enlargement. Early damage occurs in the entorhinal cortex (Arnold et al., 1991; Braak and Braak, 1991; Gómez-Isla et al., 1996), the posterior aspect of the basal nucleus of Meynert (which has strong projections to the temporal lobe; Whitehouse et al., 1991), the amygdaloid nuclei (Cuénod et al., 1993; Scott et al., 1993), and the CA1/subiculum zone of the hippocampal formation (West et al., 1994). These disease-induced changes in structure often escape detection, because of the overlap between structural changes seen in normal aging and dementia (Friedland and Luxenberg, 1988). Controversy still exists as to whether aging and AD are dichotomous, or represent a neuropathological continuum (Coleman and Flood, 1987; West et al., 1994).
Callosal Anatomy. In view of the anatomically-specific progression of cortical atrophy associated with Alzheimer's Disease, interest has focused on several questions which relate to the corpus callosum. The first question is whether local or diffuse atrophy of bilaterally connected brain regions might induce secondary effects on homotopically distributed fibers in the callosum. The second question is whether distinct regions of callosal anatomy undergo selective changes in different sub-types of dementia, and whether these changes reflect observed patterns of neuronal loss at the cortex. The third major question is whether the pervasive callosal atrophy seen late in the course of Alzheimer's Disease is preceded by a more localized pattern of fiber loss at the onset of the disease.
In one of our recent studies (Thompson et al., 1998), high-resolution 3D structural MR images were acquired from 10 subjects diagnosed with Alzheimer’s Disease according to NINCDS-ARDRA criteria (mean age: 71.9 ±10.7 yrs.; all 10 right-handed), and 10 elderly controls, matched for age (72.9 ± 5.6 yrs.), gender, educational level and handedness (all 10 right-handed). The AD patient group had a mean MMSE (Mini-Mental State Exam) score of 19.7 ± 5.7 (maximum score: 30), a rating comparable with values obtained in other dementia studies (Murphy et al., 1993), and characteristic of the early stages of the disease.The use of 1-mm thick MRI slices, combined with a high in-plane pixel resolution (0.9765mm×0.9765mm) resulted in an improved resolution imaging matrix relative to earlier studies of aging and AD pathology (e.g., slices acquired every 5 to 7-mm ).
To determine whether there was a regionally selective pattern of callosal change accompanying AD pathology, the morphology of the callosum at the mid-sagittal plane was analyzed using a 5-sector partition which is relatively simple to apply (Fig. 1(a); Duara et al. (1991); Larsen et al., 1992). This resulted in an approximate segregation of callosal fibers belonging to distinct cortical regions (see Section 2). Since individual data were standardized by digital transformation into Talairach stereotaxic space (Talairach and Tournoux, 1988), regional cross-sectional areas of callosal subdivisions were determined both before and after stereotaxic transformation. When areas of specific sectors were compared between groups, the isthmus of the callosum was of particular interest, since fibers crossing in this area selectively innervate the temporo-parietal regions at risk for early neuronal loss in AD (Brun and Englund, 1981).
Localized Atrophy. Consistent with this hypothesis, a severe and significant reduction in the area of the callosal isthmus was found in AD relative to controls, reflecting a dramatic 24.5% decrease from 98.0 ± 8.6 mm2 in controls to 74.0 ± 5.3 mm2 in AD (p < 0.025; Fig. 7(b)). By contrast, the terminal sectors (1 and 5) of the callosum, corresponding to fibers crossing in the rostrum and splenium respectively, did not undergo a significant area reduction, with almost identical values in the control and patient group of 160.9 ± 9.6 mm2 and 158.6 ± 14.3 mm2 respectively for the rostral sector (p > 0.1), and 148.7 ± 8.6 mm2 and 150.8 ± 6.8 mm2 respectively for the splenial sector (p > 0.1). An observed 16.6% mean area loss in AD for the central midbody sector showed only a trend toward significance (p < 0.1), and an apparent 13.4% depression in mean anterior midbody area was statistically insignificant (p > 0.1), because of substantial inter-group overlap in the values of these parameters in the early stages of Alzheimer's Disease.

|
|
Fig. 7. Average Boundary Representations of the Callosum in (a) Normal Elderly Subjects (N=10), (b) Alzheimer's Disease patients (N=10). Average boundary representations of the midsagittal callosum in normal controls and patients with mild Alzheimer’s Disease (MMSE ~ 19.7 ± 5.7) indicate a severe reduction in the area of the isthmus in AD relative to controls, accompanied by a pronounced inflection in shape (white arrow) in the neighborhood of stereotaxic location (0.0,-25.0,19.0). The overlying sector representing the isthmus (2nd of 5, top panel), underwent a 24.5% reduction in area in AD compared with controls (p < 0.025). |
|
|
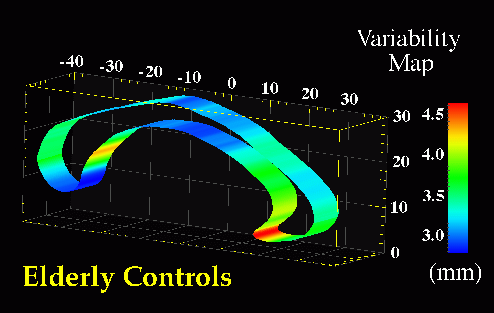
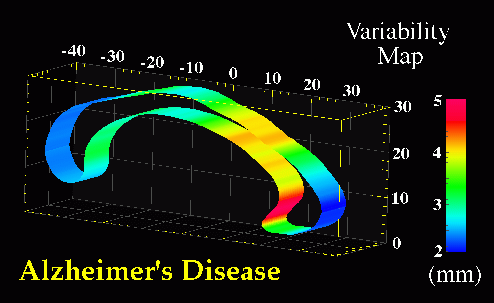
Fig. 8. Maps of Stereotaxic Variability at the Corpus Callosum in Patients with Alzheimer’s Disease and in Elderly Normal Subjects. Separate maps of local variability were constructed for (bottom) Alzheimer's patients, and (top) control subjects, expressing (in color) the r.m.s. variation of callosal points in each subject group around an average boundary representation of the callosum, at the midsagittal plane of Talairach stereotaxic space. Unlike the asymmetry and variability maps in Fig. 9, these are strictly 2-dimensional maps, since the midsagittal analysis of callosal morphology is conducted in a single vertical plane. Average boundary representations have therefore been thickened for visualization purposes only. Two trends are apparent. Although the variability measure peaks at the rostrum, this may be an artifact, due to the difficulties of defining an unambiguous anatomical limit for the inferior aspect of the rostrum. Nevertheless, the pronounced rise in variability at the rostral midbody in AD, (bottom), may reflect disease-related enlargement of the third ventricle, which forms its inferior boundary. |
Average Boundary Representations and Variability Maps. Selective changes in the CC accompanying AD pathology were examined in more detail by partitioning midsagittal sector outlines into upper and lower sectors. Average boundary representations (Fig. 7) and local variability maps (Fig. 8) were then made for the callosum in both subject groups. In both control and AD subjects, sectors showed a distinctly heterogeneous profile of variability in stereotaxic space (Fig. 8(a),(b)), with confidence limits on two-dimensional variation at the midsagittal plane varying from an SD of 2.0-3.3 mm at the inferior splenium, central midbody and genu, to 4.6-5.0 mm at the posterior aspect of the rostrum. Intriguingly, at the isthmus, where a significant area reduction was apparent in AD, the average callosal representations showed a slight reduction in thickness in AD relative to controls (Fig. 7(b)). In addition, a pronounced inflection in shape was demonstrated towards the inferior limit. This feature can be seen in Fig. 7(b), at stereotaxic location (0.0,-25.0,19.0). This morphology has also been observed in studies of callosal shape in schizophrenia (DeQuardo et al., 1996; Bookstein, 1997).
Sylvian Fissure Asymmetries. In view of the severe reduction in size at the isthmus, perisylvian areas where fibers in the isthmus originate were examined to determine whether their patterns of 3-dimensional variability and asymmetry were altered in the disease. Methods for mapping patterns of 3D cortical variation and asymmetry are discussed in detail in (Thompson et al., 1998); analysis of digital anatomic maps in normal aging and Alzheimer's Disease suggested a range of global and local disease-related differences (Fig. 9). Confidence limits on 3D cortical variation in controls showed a marked increase from 2-4 mm at the callosum to a peak of 12-13 mm at the external cerebral surface. In AD however, while variability was marginally higher than in controls at the callosal surface, the variability across the surface of the Sylvian fissure rose extremely sharply from an SD of 6.0 mm rostrally to 19.6 mm caudally on the left, and from 5.0 mm rostrally to 9.0 mm caudally on the right.
Although there is a substantial literature on Sylvian fissure cortical surface asymmetries (Eberstaller, 1884; Cunningham, 1892; Geschwind and Levitsky, 1968; Davidson and Hugdahl, 1994) and their relation to functional lateralization (Strauss et al., 1983), handedness (Witelson and Kigar, 1992), language function (Davidson and Hugdahl, 1994), asymmetries of associated cytoarchitectonic fields (Galaburda and Geschwind, 1981) and their thalamic projection areas (Eidelberg and Galaburda, 1982), no prior studies had mapped the spatial profile of asymmetries in 3 dimensional space. When Sylvian fissure asymmetries were mapped in 3 dimensions, the marked rostral and vertical extent asymmetries in controls (left posterior limit 9.7 mm more caudal than right; p < 0.0005) were severely increased in AD (left limit 16.6 mm more caudal than right; p < 0.0002), and between-hemisphere differences in the anterior-posterior position of the Sylvian fissure's posterior limit were also found to be significantly greater in Alzheimer's Disease than in matched controls (p < 0.05). Local maps (Fig. 10) also revealed a sharp rise in 3-dimensional asymmetry in AD, reaching 21.8 mm at the posterior limit of the left Sylvian fissure, compared with 15.4 mm in controls.
Asymmetric Progression of Alzheimer's Disease. Greater variability of the left perisylvian surface and greater Sylvian fissure asymmetry in AD suggests that AD pathology asymmetrically disrupts the anatomy of temporo-parietal cortex. In AD, Sylvian fissure CSF volume has also been shown to rise, relative to controls, more sharply on the left than the right (left volume: 31.5% higher in AD than controls (p < 0.02; N=32) but right volume only 20.4% higher (p < 0.09; Wahlund et al., 1993). Underlying atrophy and possible left greater than right degeneration of perisylvian gyri may widen the Sylvian fissure, superimposing additional individual variation and asymmetry on that already seen in normal aging (Fig. 9). Significant left greater than right metabolic dysfunction and cognitive impairment have been reported in both PET (Loewenstein et al., 1989; Siegel et al., 1996) and neuropsychiatric (Capitani et al., 1990) studies of AD, and in PET studies of related amnestic disorders (Corder et al., 1997). Recent PET studies have also examined cognitively normal subjects who are 'at risk' for AD in the sense that they experience mild memory complaints and have at least two relatives with AD (Small et al., 1995). Left greater than right metabolic impairment also appears in these subjects, and the deficit has been shown to be significantly more asymmetric in at-risk subjects carrying the apolipoprotein type 4 allele (ApoEe 4; a risk factor for familial AD) relative to those subjects without ApoEe 4 (Small et al., 1995). Accentuated patterns of structural asymmetry and variation found here in Alzheimer's Disease support these findings, suggesting either that (1) the left hemisphere is more susceptible than the right to neurodegeneration in AD, or that (2) left hemispheric impairment results in greater structural change and lobar metabolic deficits (Loewenstein et al., 1989).
Callosal Deficits in Alzheimer's Disease. An anatomically-specific relationship was observed in this and other studies (Lyoo et al., 1997) between putative fiber loss in callosal regions and the dynamic progression of cortical and lobar atrophy characteristic of AD. Size reduction and shape inflection were observed in callosal regions that map association areas at risk of selective metabolic loss and incipient atrophy in early stages of AD (Figs. 4 and 6). Greater resilience was observed in splenial sectors, which map the parieto-occipital and calcarine surfaces which are comparatively resistant to neuronal loss in AD (Pearson et al., 1985). Severe and regionally-selective areal loss and focal shape inflection at the isthmus may reflect disease-related disruption of the commissural system connecting bilateral temporal and parietal cortical zones, since these regions are known to be at risk for early metabolic dysfunction, perfusion deficits and selective neuronal loss in AD (DeCarli et al., 1996).
Differences between Normal Aging and Dementia. As noted earlier, controversy exists over whether different callosal regions undergo selective changes in aging and dementia (Yoshii et al., 1990), and even whether effects in dementia are significant compared to age-matched controls (Biegon et al., 1994). As found in Biegon et al. (1994), Black et al. (1996) and Kaufer et al. (1997), we did not find total callosal area to be significantly depressed in AD (mean ± SD: 525.9 ± 116.8 mm2) relative to controls (575.4±108.8 mm2; p > 0.05). These values (determined prior to stereotaxic normalization) are in broad agreement with, but marginally higher than, previously determined callosal area values (Biegon et al., 1994) of 562 ± 98 mm2 in elderly controls (n=13; MR slice thickness: 10 mm; age: 64.8 ± 9.0 yrs.) and 480 ± 133 mm2 in AD (p > 0.05; n=20; age: 70.1 ± 7.4 yrs., with slightly lower MMSE scores of 17 ± 7.2, and a range of 9-25). Both our data and those of Biegon et al. (1994), Black et al. (1996) and Kaufer et al. (1997) do not fully agree with earlier reports that total callosal area discriminated between AD and control subjects (Hofmann et al., 1995; p < 0.05), and that combined callosal area was significantly reduced in AD (by 7.0% relative to controls; p < 0.05; Yoshii et al., 1994). If selective AD-related atrophy occurs in specific callosal sectors (Black et al., 1996), when areas of these sectors are pooled with that of other more robust regions, they may or may not be significantly reduced, depending on disease severity.
Alzheimer's Disease and Schizophrenia. The finding that the same highly circumscribed site at the callosum (Fig. 8) shows a comparable shape inflection in schizophrenia (DeQuardo et al., 1996; Bookstein, 1997) is intriguing. Due to differences in the etiology of the two diseases, fiber deficiency at this site in schizophrenia may be associated with a neurodevelopmental disruption of the sulco-gyral organization of temporal lobe fibers that cross in this region (see Section 8; Kikinis et al., 1994). In AD, however, a similarly-localized fiber deficiency may result from temporo-parietal neuronal loss, associated with the early perfusion and cognitive performance deficits in AD. As noted already, a massive perinatal loss of callosal axons, lasting from the 35th gestational week to the end of the first post-natal month (Clarke et al., 1989; La Mantia and Rakic, 1990) leads to a restricted pattern of adult callosal connections, but controversy exists over whether callosal area is further reduced in normal aging relative to young normal controls (Biegon et al., 1994; cf. Doraiswamy et al., 1991). Callosal area reductions in AD may have functional significance, as smaller partial callosal size often reflects a focal decrease in the number of small diameter (< 3 µm) fibers (Aboitiz et al., 1992) or decreased myelin deposition, associated with decreased conduction velocity and longer interhemispheric transmission times. These and other callosal studies, interpreted in the context of (1) the temporo-parietal perfusion deficits and temporal neuronal loss typical in early AD, and (2) correlations between reduced association cortex metabolism and cognitive performance, suggest that neuronal loss and white matter abnormalities in AD may partially exert their effect through disruption of long cortico-cortical pathways (DeCarli et al., 1996). Earlier reports of CC alterations in clinically mild AD (Vermersch et al., 1994; Hoffman et al., 1995; Janowsky et al., 1996), support these observations and further highlight the selective vulnerability of callosal regions in AD.
Other Dementia Subtypes. Recent reports suggest that the pattern of callosal atrophy may differ in different subtypes of dementia, at least in the early stages of each disease. Using the Witelson partition (1989; Fig. 1(c)), Lyoo et al. (1997) observed that mildly demented (MMSE > 21) subjects with Alzheimer's Disease exhibited significant area reductions at the posterior midbody, isthmus and splenium (N=49; Fig. 1(c)), while subjects with mild vascular dementia (according to DSM-III and Hachinski diagnostic criteria, and with MMSE > 21; N=21) had only a significantly smaller genu relative to normal controls (N=36). This finding is consistent with prior reports that the anterior vasculature is preferentially affected in vascular dementia (Freedman and Albert, 1985; Ishii et al., 1986). Observed post mortem, patients with vascular dementia also have a reduced axon density specific to the anterior callosum (Yamanouchi et al., 1990; N=5). Nonetheless, all callosal sectors exhibit significant size reductions in the more severe stages of both diseases (MMSE < 21; Lyoo et al., 1997). In view of the distinct etiologies of different types of dementia, further neuroimaging studies are required to clarify the relation between structural and functional deficits throughout the dynamic course of each disease.
VIII. Schizophrenia
Callosal Morphometry. People with schizophrenia exhibit subtle and diffuse alterations in cerebral structure as revealed by evidence from post mortem and in vivo imaging studies. None of these neuroanatomical abnormalities, however, have been shown to be requisite for diagnosis (Stevens, 1998). Nevertheless, technological advances in structural and functional imaging protocols and improvements in quantitative methods for measuring brain morphology and activation continue to increase the sensitivity with which deviation of neuroanatomic and functional norms can be detected. In spite of novel imaging techniques and applications, morphometric findings in schizophrenic populations frequently lack consensus and fail to expose reliable patterns of structural variation within and across schizophrenia subtypes compared to normal. In fact, the reported morphometric differences appear to be as variable as patient symptomatology. Imaging studies designed to detect macroscopic alteration of callosal morphology have yielded mixed results. There is however a fair amount of experimental evidence suggesting that transcallosal connectivity plays an important role in the schizophrenia syndrome. Furthermore, schizophrenia fits the profile of a misconnection syndrome, reflecting dysfunctional callosal connections from homologous regions of cortex, especially those between the sometimes asymmetric areas of association cortex (Crow, 1998). In the following sections we will discuss: (1) how maldevelopment of the callosum may relate to other structural neuropathology reported in schizophrenia; (2) theoretical issues that relate symptomatology in schizophrenia to callosal abnormality; (3) empirical evidence from neuroimaging and neuropsychological studies suggesting structural alterations of the callosum and related behavioral deficits; and finally (4) methodological issues related to detecting structural abnormalities of the callosum in this population.
Callosal Links to Structural Neuropathology in Schizophrenia. Diffuse structural abnormalities have been reported in neuroimaging, cytoarchitectural, epidemiological and behavioral studies of schizophrenic populations. Converging evidence supports the idea of schizophrenia as a circuit disease, stemming from aberrant neurodevelopmental events (e.g., Carpenter et al., 1993; Pilowsky et al., 1993; Waddington et al., 1993; Nopoulos et al., 1995; Weinberger et al., 1995; Buchanan, 1997). The disruption of neurodevelopmental events involving mechanisms such as abnormal neuronal migration, growth retardation, aberrant myelination, neuronal pruning and/or apoptosis occurring prenatally or through development are thought to account for the diversity of structural findings in schizophrenia (Waddington et al., 1993; Nopoulos et al., 1995). The fact that pruning and myelination in the corpus callosum continue into early adulthood and that interhemispheric coherence develops up until late adolescence may also have some relevance to the age of onset (Njiokiktjien et al., 1994). Furthermore, myelination begins prenatally and has been shown to be susceptible to malnutrition, asphyxia, exotoxins and endotoxins of infectious origin (Njiokiktjien et al., 1994; Bishop and Wahlsten, 1997). It may be no coincidence that influenza epidemics or other viral infections during the second trimester, a critical period for neurodevelopment and obstetric complications, lead to an increase in schizophrenic births (Swayze et al., 1990; Waddington et al., 1993, Woodruff et al., 1995).
A clearer picture of callosal dysfunction in schizophrenia emerges by considering structural abnormalities in the context of their functional circuitry. After all, no brain region acts in isolation and alterations in circuitry potentially lead to structural abnormalities, and vice versa. In spite of primary confounds such as schizophrenia heterogeneity and different methodological approaches, there are some suggestive neurodevelopmental abnormalities frequently reported in schizophrenia populations involving neural systems linked by the callosal commissure. The major players include frontal, temporo-limbic and midline circuits (Cassanova, 1990; Andreasen et al., 1994, 1996; Bogerts, 1997; Goldman-Rakic and Selemon, 1998). As the primary channel of interhemispheric communication, the corpus callosum plays an important role in these systems by reciprocally connecting homologous regions of neocortex. This means that an abnormality in prefrontal or temporal cortices, for example, is likely to influence callosal morphology, and a disturbance in callosal development is likely to have reciprocal affects on these cortical regions.
Callosal Development in Schizophrenia. As noted earlier, the development of the corpus callosum and the neopallium occurs in parallel, with the embryonic callosum first connecting nonolfactory cortex, then extending anteriorly to connect frontal cortices, and then posteriorly to join other lobes as they develop. Recent evidence, however, suggests that the callosum develops bidirectionally (Kier et al., 1996; see Section 4). Nevertheless there is no question that the corpus callosum develops in the hippocampal primordium in close relationship with the developing lobes.
Imaging studies indicate that median reductions in prefrontal cortex make these regions about 5.5% smaller in schizophrenic patients. Reductions are primarily due to a loss in gray rather than white matter (Lawrie and Abukmeil, 1998). Superior and rostral portions of the hippocampal formation also undergo regressive changes in association with callosal development. While findings regarding volume decreases in limbic structures remain equivocal in schizophrenic patients, results in homogeneous samples (males with positive symptoms) are more conclusive (Shenton et al., 1992; Flaum et al., 1995; Menon et al., 1995; Barta et al., 1997; Marsh et al., 1997). Morphological alteration of the superior temporal gyrus in schizophrenic patients has also been reported (e.g., Nestor et al., 1993). This gyrus has particular relevance to callosal circuitry, because it has been identified as the approximate site of Wernicke's language area, and marks the planum temporale on its superior banks (Geschwind et al., 1985; Galaburda et al., 1990). To focus on language-related cortex in schizophrenic patients, investigators have measured the asymmetry of the planum temporale and Sylvian fissure in MR images. Once again, results are mixed with some studies reporting a decreased asymmetry in schizophrenic patients (Hoff et al., 1992; Kikinis et al., 1994; Petty et al., 1995) and others not (De Lisi et al., 1994; Kleinschmidt et al., 1994; Frangou et al., 1997). While corpus callosum morphometry and planum asymmetry have not been compared in the same schizophrenic sample, data from normal populations indicate that morphology in these areas are related (Aboitiz et al., 1992). In summary, imaging studies examining temporal and limbic structures report a modest reduction in temporal lobe volume, with larger volume reductions in the hippocampus, amygdala and superior temporal gyrus, and some reports of abnormal asymmetries of the planum temporale and Sylvian fissure.
Towards the end of embryonic callosal development the area between the corpus callosum and the fornix becomes very thin, and forms the septum pellucidum, where the cavum septi pellucidi may develop. MRI and post mortem studies have related presence of cavum septi pellucidi to psychosis. This developmental anomaly is especially prevalent in schizophrenia groups, and relates to other schizophrenia structural abnormalities (Degreef et al., 1992; Nopoulos et al., 1996). In addition, the corpus callosum forms the rostral boundary and roof of the superior horn of the lateral ventricles. Cerebral ventricular enlargement is the most robust structural anomaly reported in schizophrenic patients. Median figures from many studies indicate the most substantial enlargement is in the ventricular body (Lawrie and Abukmeil, 1998). While finding no difference in corpus callosum area, thickness, or length in twins discordant for schizophrenia, Cassanova et al. (1990) used a statistical analysis of a Fourier expansion series to demonstrate differences in callosal shape between discordant twins. The shape difference was especially apparent in the middle and anterior segments of the callosum. The displacement of the corpus callosum in the vertical plane of schizophrenic patients, representing a shape difference between populations has been replicated, and reflects the increased size of the lateral ventricular body (Narr et al., unpublished findings). Finally, thalamic abnormalities along with other midline structure alterations such as cavum septum pellucidi (Nopoulos et al., 1996) and increased incidence of callosal agenesis (Swayze et al., 1990) suggests the role of neurodevelopmental abnormalities and the disruption of midline circuitry in schizophrenia.
Callosal Connectivity in Schizophrenia. Considering the major role the corpus callosum plays in hemispheric transmission, it is conceivable that the corpus callosum must be involved in schizophrenia neuropathology. Crow (1998) proposes that schizophrenic etiology arises from a component of callosal connectivity associated with the evolution of language, arguing that language and psychoses have a common evolutionary origin. He supports this view by pointing out that age of onset, relative constancy of incidence across societies, and affliction during reproductive and healthy phases of life are characteristics of schizophrenia, and this disease may not be selected out because of its association with language specialization. While this misconnection hypothesis is difficult to test, evidence is cited of abnormalities in the "last to evolve" fiber pathways (for example, perisylvian temporal and dorsolateral prefrontal cortex) and reports of callosal morphologic change over time in schizophrenic patients. Additionally, some studies have reported a link between schizophrenia and dyslexia, representing schizophrenia etiology as tied to language lateralization and callosal connectivity (Stein, 1994; Horrobin et al., 1995). There are however studies that directly dispute the misconnection hypothesis; for example, Bartley et al. (1993) found no evidence of altered asymmetry in language areas in monozygotic twins discordant for schizophrenia, and other studies have not found altered planum asymmetry in schizophrenic patients (De Lisi et al., 1994; Kleinschmidt et al., 1994; Frangou et al., 1997).
David (1994) discusses different views regarding how abnormal interhemispheric transmission accounts for typical schizophrenic phenomena, such as hallucinations and thought alienation. One view suggests that cognitive activity within the right hemisphere would be experienced as alien by the left hemisphere if the two hemispheres were not in full communication (Nasrallah et al., 1985). Another model of interhemispheric abnormality in schizophrenia assumes that excessive connectivity between the two hemispheres, perhaps due to insufficient pruning during development, causes confusion as to whether stimuli are of external or internal origin (Randall, 1983). Clinical studies have also reported cases of psychosis in patients with complete or partial callosal agenesis (David, 1994). As mentioned above, callosal agenesis and other callosal abnormalities are thought to have increased incidence in schizophrenia (Swayze et al., 1990; Degreef et al., 1992; Nopoulos et al., 1996).
Post Mortem and Imaging Studies. The most direct evidence of structural callosal abnormalities in schizophrenia comes from post mortem and imaging studies. Experimental neuropsychology also contributes with specific behavioral measures designed to assess impairments in interhemispheric communication implicating callosal abnormality. Findings of altered callosal morphology in schizophrenic patients appear complex and tempered by a number of variables including sex, symptoms, disease course and age of onset. In fact, there appears little consensus on whether the corpus callosum is larger or smaller in this population. In spite of conflicting evidence, a meta-analysis by Woodruff et al. (1995) found a modest reduction of callosal size across 11 studies with heterogeneous schizophrenic samples. To complicate matters further, structural differences are confounded by employment of different measurement techniques, making it difficult to compare results across studies. If one thing is clear from this body of research, it is that the story of callosal abnormality in schizophrenia is as complicated as other facets of the disease.
Early post mortem studies report an increase in callosal size in schizophrenia (Bigelow et al., 1983), but this finding has not always been replicated (Brown et al., 1986). Advances in imaging procedures have made it easier to study the corpus callosum in vivo but studies continue to report mixed results for different callosal parameters. For example, some studies have found total callosal area to be smaller (e.g. Rossi et al., 1989; Woodruff et al., 1993), larger (e.g., Nasrallah et al., 1986) or not significantly different (e.g., Uematsu and Kaiya, 1988) in schizophrenic patients compared to controls. Other studies measuring callosal thickness have similarly reported increases (e.g. Nasrallah et al., 1986), decreases (e.g., DeQuardo et al., 1996), and no difference across groups. As discussed earlier (Section 2), efforts have been made to parcellate the callosum into sections, to isolate malformation of specific callosal channels (see Fig. 1). Here again results conflict, perhaps in part due to variations in methods, and the different schizophrenia subgroups tested. For example, roughly equivalent estimates of the anterior corpus callosum connecting frontal cortices have been reported as thicker or larger in area (Nasrallah et al., 1986; Uematsu and Kaiya, 1988), smaller in area (Woodruff et al., 1993, Jacobsen et al., 1997) or not different (Woodruff et al., 1997) in patients. Posterior and middle callosal regions in schizophrenic patients have also been found reduced (DeQuardo et al., 1996; Woodruff et al., 1993, Jacobsen et al., 1997), thicker (Nasrallah et al., 1986) or to have similar distributions with normal controls (Smith et al., 1987; Uematsu et al., 1988; Woodruff et al., 1997).
Gender Interactions. Results mentioned above and elsewhere indicate an incredible range of callosal morphometric findings in schizophrenic populations. Although failure to replicate findings may be attributable to the heterogeneity of the patient groups examined, discrepancies may also result from structural differences between sexes in schizophrenic groups. As noted in Section 3, there is an ongoing controversy concerning sex differences in callosal structure, and it appears that brain size has a larger influence over callosal morphology than does gender (Rauch et al., 1994; Jäncke et al., 1997; see Section 3). Larger callosal size found in females relative to brain or skull size results directly from their smaller brain size, and this relative difference persists irrespective of sex. Even if no sex difference is present in normal callosal morphology (cf. Bishop and Wahlsten, 1997), this does not preclude sex effects from interacting with morphometric abnormalities in schizophrenic populations. Male and female schizophrenics may, in general, follow a different course of illness. There is typically a later age of onset in female schizophrenics and hereditary factors may be unevenly distributed between the sexes (De Lisi et al., 1989; Waddington, 1993). Callosal structural alterations in schizophrenic patients may therefore reflect gender differences or gender interactions. Hoff et al. (1994), for example, found that females with first episode schizophrenia had smaller total callosal area compared to controls. These findings partially replicated data from a study by Hauser et al. (1989) where chronic female schizophrenic patients showed smaller anterior callosal widths compared to normals. In contrast, Nasrallah found increased thickness of the anterior and middle corpus callosum in females, while Raine et al. (1990) found increased thickness in the callosum of female schizophrenics compared to normal females and decreased callosal thickness in male patients compared to male controls. A similar trend towards a ‘reversed’ sex difference in anterior and posterior callosal size was reported by Colombo et al. (1993).
Clinical Variables. Clinical and psychopathological heterogeneity in schizophrenic patients may account for inconsistencies in results when assessing structural morphology (Colombo et al., 1993). For example, patients with negative symptoms are shown to have smaller callosal sizes relative to patients with positive symptoms or to controls (Gunther et al., 1991; Woodruff et al., 1993). It has also been suggested, however, that patients with negative symptoms have thicker corpus callosums (Coger and Serafetinides, 1990; Jacobsen et al., 1997). Early onset schizophrenic patients have been shown to have larger total, anterior and posterior callosal areas as compared to controls (Coger and Serafetinides, 1990; Jacobsen et al., 1997). These results are consistent with the post mortem data of Bigelow et al. (1983) that found early onset chronic schizophrenic patients to have significantly greater callosal thickness than later onset patients. In addition, auditory hallucinations have been related to callosal size (David et al.,1995). Lastly, one study suggested that an elongated anterior callosum related to unfavorable prognosis, because large callosal size suggested poor heterosexual relations, reduced numbers of hospitalizations, low academic grades and mild anxiety-depression syndrome (Uematsu and Kaiya, 1988). Clearly, more information is needed to establish the role of these factors in relation to callosal morphometry in schizophrenia. Finally, almost all studies used solely right-handed subjects in their analyses, or matched for handedness across groups. Controlling for handedness is important since handedness appears a predictor of neuroanatomic asymmetry, and bears a relationship to callosal morphology (Bartley et al., 1993; Clarke and Zaidel, 1994). Most studies also reported no influence of age in their findings of callosal pathology. Even though the effects of aging and callosal development are still under investigation (Sections 4-6), these results may not be surprising as most studies controlled for age in some degree and used primarily adult samples.
Structure/Function Correlations. To assess the relationship between callosal structure and function in schizophrenia, several investigators correlated measures of interhemispheric transfer and hemispheric specialization with callosal morphometry (Raine et al., 1990; Colombo et al., 1993; Woodruff et al., 1996). Tests of interhemispheric transfer include measures of visual, tactile, auditory, and cognitive transfer. Such tests have been derived from studies assessing the absence of callosal relay in split-brain patients. Patients with surgical transection of the corpus callosum exhibit impairments such as left hand anomia in tactile tasks, inability to identify false speech sounds presented to the right ear, and deficits in matching stimuli across visual fields (Gazzaniga, 1995). Similar impairments in schizophrenic patients would therefore imply abnormalities in callosal relay. Raine and colleagues (1990) were one of the first groups to employ concurrent structural and functional callosal measures in a schizophrenia sample. Behavioral measures included verbal and nonverbal dichotic listening protocols and crossed versus uncrossed conditions in finger sequence repetition, tactile and WAIS-R block design tasks. None of these tasks detected deficits in interhemispheric processing in patients, and attempts were not made to correlate scores with the sex interaction of callosal morphometry detected across groups. A neuropsychological battery with unilateral and bilateral conditions employed by Hoff et al. (1993) revealed an association of larger callosal size with better cognitive function but no relationship was noted in female schizophrenic patients who exhibited significantly smaller callosal area or across genders in the schizophrenic group. Similar non-significant results of interhemispheric transfer have been reported in studies assessing behavioral measures alone (e.g., Schrift et al., 1986; Ditchfield et al., 1990). In another of the few studies simultaneously assessing structural and functional abnormality of the callosum in schizophrenic patients there were no deficits reported in interhemispheric transfer for an auditory comprehension task assessing transfer of speech information (Colombo et al., 1993). However, the degree of left versus right ear response in the monaural condition in this paradigm was significantly correlated with posterior callosal size in male schizophrenics.
Color Naming and Matching. Although studies assessing interhemispheric transfer have yielded unpromising results in schizophrenic patients, more interesting results have stemmed from studies assessing color naming and matching. Here patients show impairments in left visual field color naming, reflecting impairments in callosal transfer. Impairments are greater in across field color matching relative to within-field matching (McKeever et al., 1979; David, 1989). In a similar vein, a lateralized version of the color-word Stroop task administered to schizophrenic patients revealed that the reaction time difference across visual fields for congruent and incongruent color word pairs was increased (David et al., 1994). This suggests a hypoconnection of facilitation and interference between hemispheres. Using a version of the color-word Stroop task, Woodruff and colleagues (1997) found that bilateral facilitation was greater and interference less in schizophrenic patients. Furthermore, these investigators included simultaneous morphometric analysis of the corpus callosum in their sample. However, they found a positive correlation between facilitation and posterior callosal area, and a complimentary negative correlation between interference and posterior callosal area only when both patient and control groups were combined.
In general, tests of interhemispheric transfer in schizophrenic patients have yielded disappointing results as far as a misconnection hypothesis is concerned. Studies including morphometric measures and those assessing behavioral measures alone have so far yielded no consistent deficit in interhemispheric transfer specific to this population but instead have indicated a more particular left hemisphere deficit (Schrift et al., 1986; Ditchfield et al., 1990). Functional imaging studies, however, have revealed decreased activity in the corpus callosum in disorganized schizophrenic patients (Schroder et al., 1995). Evidence of different regional cerebral activation patterns in each clinically defined subgroup serves to emphasize the importance of studying homogeneous patient groups. Bilateral hyperactivation has also been reported in schizophrenic patients with primarily positive symptoms in a sensorimotor task and this subgroup also exhibited increased callosal size (Gunther et al., 1991). Many methodological problems however, occur in research assessing interhemispheric communication. Until more evidence is obtained, especially in regard to measuring structural and functional correlates, no solid conclusions can be drawn about interhemispheric impairment in schizophrenic patients.
Methodological Issues. Morphometric analysis of structural differences in schizophrenic brains from post mortem and in vivo imaging studies is a relatively dirty task. That is, many extraneous variables interact to threaten validity of results. Since the schizophrenia syndromes may arise from multiple pathophysiological mechanisms it is not always clear how to reduce patient heterogeneity when examining particular and connected morphometric anomalies. As indicated above, differences in sex, chronicity, symptomatology, age of onset, handedness and age may all influence callosal morphology in schizophrenia groups. Other factors that may interfere with analysis include the incidence of perinatal insult, educational level, IQ, height, social status, premorbid adjustment, treatment history and response, and the duration and course of illness (Carpenter et al., 1993). Further difficulties arise from the large variability present in normal populations regarding callosal structure and the significant overlap with patient distributions. Interactions of these variables increasingly complicate the endeavor to examine structural alteration of the corpus callosum and its relationship with other neuroanatomic abnormalities in schizophrenia.
Some solutions to these problems may include the ability to compare images from individual patients with those of an appropriately matched average from normal brain archives. This is now becoming possible, as a probabilistic reference system for the human brain is being compiled whereby variables such as age, sex and handedness may be matched (Mazziotta et al., 1995; Thompson et al., 1997, 1998; see Section 9). Correcting for head size differences appears all-important when studying the nature of callosal structural neuropathology (Steinmetz et al., 1995; Jäncke et al., 1997), especially in view of sex differences in head size. Furthermore, while schizophrenic patients are shown to have decreased cranial and cerebral size, that does not necessarily hold true for other cerebral structures. To illustrate this point, no callosal structural abnormalities were revealed in childhood onset schizophrenic patients (Jacobsen et al., 1997). However, after adjusting for the smaller cerebral volume of the schizophrenics, larger total, anterior and posterior corpus callosum areas were detected in patients. Unfortunately not all studies assessing callosal structure abnormalities in schizophrenia have adjusted for brain size. Transforming images into Talairach standardized space (Talairach et al., 1967; Talairach and Tournoux, 1988) or correcting for both measured cortical volumes and head positioning in the MR scanner are suggested to allow for proper morphometric comparison of neuroanatomical regions across populations.
Patient Homogeneity. In a meta-analysis of heterogeneous schizophrenic patients, Woodruff and colleagues (1995) noted the huge variability in magnitude and direction of callosum size in the patient groups. To address the problem of patient heterogeneity, the most simple solution is to study homogeneous groups. Unfortunately, this has a trade-off with the ability to generalize results. Some investigators suggest that besides matching for demographic variables either the type of course of illness or the evaluation of outliers should be used when assessing morphology in schizophrenia brains (Stevens, 1998). Others argue that neuroanatomy should be examined in the context of separate symptom complexes that may or may not alter regional anatomy (Carpenter et al., 1993). Furthermore, as noted in Section 3, large sample sizes are required to assess the relationships of structural brain abnormalities as exist in neural circuits, especially as callosal abnormality in schizophrenia appears to have a small to medium effect size (Woodruff et al., 1995).
Results are further complicated by different protocols used to divide the callosum into sub-regions (see Fig. 1) and in the measures used to obtain maximal or minimal callosal thickness (see Fig. 1(d)). Even though research in monkeys shows that the corpus callosum is organized by sensory modality (Pandya & Seltzer, 1986), there are no neuroanatomical landmarks by which to partition these areas. While some investigators have investigated how different partitioning protocols influence validity of results (e.g., Allen et al., 1991), different methods make it difficult to compare results across studies. Furthermore, most analyses have only looked at traditional morphometric parameters that include midsagittal area, maximum and minimum width and length (Fig. 1). More recent techniques allow the analysis of curvature, shape and fractal complexity (Thompson et al., 1996a,b). Methods which involve the creation of parametric meshes rendered from traces of regional surface anatomy enable the visualization of very local variability and group differences across the surfaces of individual structures (Thompson et al., 1996, 1997; see Section 9). Additionally, even fewer studies have related callosal morphometric differences to data from other brain regions. Since callosal function depends on the integrity of the cortical regions it connects, it does not make sense to study this region in isolation. Tissue segmentation protocols indicate a gray matter volume loss in the cortices of schizophrenic patients (e.g., Lim et al., 1995; Sullivan et al., 1996). Considering the mixed findings of callosal abnormality it is still not clear whether white matter sparing in schizophrenia includes the traversing callosal fibers or whether disconnection or misconnection occurs. Finally, only one study to our knowledge has looked at changes in callosal morphology in schizophrenic patients across time (De Lisi et al., 1995).
There are also difficulties in interpreting the nature of interhemispheric deficits in schizophrenic patients and their relationship to callosal structure. Part of the problem appears because even in normals many behavioral tests of interhemispheric transfer have often not been found to be related to measurements of callosal structure (Clarke and Zaidel, 1994). In post mortem studies, Aboitiz et al. (1992) noted that only the numbers of small diameter fibers thought to relay cognitive information across hemispheres relate to callosal size while large diameter fibers conducting sensory and motor information do not. Considering that most of the interhemispheric tasks employed in the schizophrenia studies reviewed involved mainly the transfer of sensory information (visual, tactile and auditory transfer), it may not be surprising that these measures did not correlate well with callosal structural abnormalities, although abnormalities were detected in imaging activation tasks (Gunther et al., 1991; Schroder et al., 1995). Also, many trials are required to assess small differences in reaction time for stimuli presented within versus between hemispheres. In these types of behavioral paradigms, results may also be compromised by patient distractibility. Finally, the fine temporal resolution of EEG studies augments the power to detect differences in processing time across the hemispheres, and these studies have reported abnormal interhemispheric transfer time in schizophrenic patients (e.g., Weisbrod et al., 1997; Heidrich and Strik, 1997).
The large body of research dedicated to studying callosal abnormality in schizophrenic patients at the structural and behavioral levels has thus far been unable to reach a consensus. As improvements in imaging analysis techniques provide enhanced detection of subtle differences, and as more is learned about callosal structure and function in normal populations, a clearer picture of callosal abnormality in schizophrenia is likely to emerge.
IX. Atlas-Based Pathology Detection
Probabilistic atlasing is a research strategy whose goal is to generate anatomical templates that retain quantitative information on inter-subject variations in brain architecture (Mazziotta et al., 1995; Thompson et al., 1997; Thompson and Toga, 1998). A digital probabilistic atlas of the human brain, incorporating precise statistical information on positional variability of important functional and anatomic interfaces, may help to address many of the methodologic difficulties we have observed in detecting alterations in callosal anatomy. As the database of subjects on which probabilistic atlases are based increases in size and content, the digital, electronic form of the atlas provides efficiency in statistical and computational comparisons between individuals or groups. In addition, the population on which probabilistic atlases are based can be stratified into subpopulations by age, gender, handedness, or other demographic factors, by stage of development or to represent different disease types. A probabilistic framework for structural brain data solves many of the limitations of a fixed atlas in representing highly variable anatomy. A statistical confidence limit, rather than an absolute representation of neuroanatomy may also be more appropriate for representing particular subpopulations.
Warping Algorithms can Create Probabilistic Atlases. As noted earlier, when applied to two different 3D brain scans, a non-linear registration or warping algorithm calculates a deformation map (Fig. 2,3) which matches up brain structures in one scan with their counterparts in the other. The deformation map indicates 3-dimensional patterns of anatomic differences between the two subjects. By defining probability distributions on the space of deformation transformations which drive the anatomy of different subjects into correspondence (Grenander, 1976; Amit et al., 1991; Grenander and Miller, 1994; Thompson and Toga, 1997; Thompson et al., 1997), statistical parameters of these distributions can be estimated from databased anatomic data to determine the magnitude and directional biases of anatomic variation. Encoding of local variation can then be used to assess the severity of structural variants outside of the normal range, which, in brain data, may be a sign of disease (Thompson et al., 1997).
|
Fig. 9. Population-Based Maps of 3D Structural Variation and Asymmetry. Statistics of 3D deformation maps can be computed to determine confidence limits on normal anatomic variation. 3D maps of anatomic variability and asymmetry are shown for 10 subjects with Alzheimer's Disease (age: 71.9±10.9 yrs.), and 10 normal elderly subjects matched for age (72.9±5.6 yrs.), gender, handedness and educational level (Thompson et al., 1998). Normal Sylvian fissure asymmetries (right higher than left; p<0.0005), mapped for the first time in 3D, were significantly greater in AD than in controls (p<0.0002; top panels). In the 3D variability maps derived for each group (lower panels), the color encodes the root mean square magnitude of the displacement vectors required to map the surfaces from each of the ten patients' brains onto the average. Confidence limits on 3D cortical variation (lower right panel), exhibited severe increases in AD from 2-4 mm at the corpus callosum to a peak standard deviation of 19.6 mm at the posterior left Sylvian fissure. |
Encoding Brain Variation. The random vector field approach is a general strategy to construct population-based atlases of the brain (Thompson and Toga, 1997; Thirion et al., 1998). Briefly, given a 3D MR image of a new subject, a warping algorithm (Thompson and Toga, 1996) calculates a set of high-dimensional volumetric maps, elastically deforming this image into structural correspondence with other scans, selected one by one from an anatomic image database. Target scans are selected from subjects matched for age, handedness, gender, and other demographic factors (Thompson et al., 1997, 1998). The resulting family of volumetric warps provides empirical information on patterns of local anatomic variation. A probability space of random transformations, based on the theory of anisotropic Gaussian random fields (Thompson et al., 1997), is then used to encode the variations. Specialized continuum-mechanical approaches are required to encode information on complex variations in gyral and sulcal topography from one individual to another (Thompson et al., 1997; Thompson and Toga, 1998). Confidence limits in stereotaxic space are determined, for points in the new subject's brain, enabling the creation of color-coded probability maps to highlight and quantify regional patterns of deformity.
Callosal Anatomy. Although probabilistic systems are under development for the population-based analysis of callosal data (Thompson et al., 1998), their ability to resolve callosal differences in small groups of subjects with dementia is indicated in Fig. 7. In one validation experiment (Thompson et al., 1997; Fig. 10), probability maps were created to highlight abnormal deviations in the callosal anatomy of a specific subject. The medical history of the subject in question indicated the presence of lung cancer, which had spread to the brain. Two metastatic tumors were present, one in each hemisphere. The first, and larger, tumor (volume: 95.2 cm3; see Fig. 10) was centered in the high putamen of the right hemisphere, while a second tumor (volume: 24.6 cm3) was located in the left occipital lobe. No other neuropathology was present, and the primary cause of death was cardiopulmonary arrest. The two regions of metastatic tissue induced marked distortions in the normal architecture of the brain. This effect was reflected both in the blockface imagery itself (Fig. 10, top) and in the values of the probability maps of structures proximal to the lesion sites (Figs. 10, bottom).
 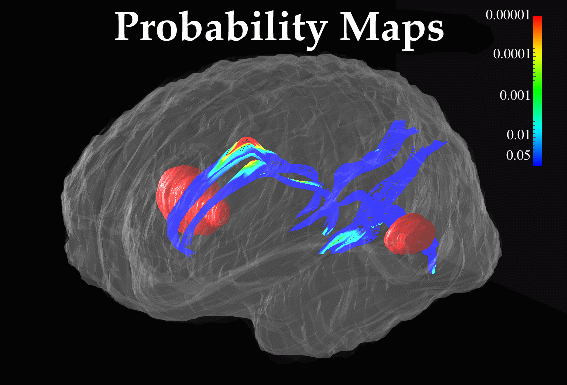
|
|
Fig. 10. Distortions in Brain Architecture induced by Tumor Tissue: Probability Maps for Ventral Callosum and Major Sulci in Both Hemispheres. Color-coded probability maps, (lower panel), quantify the impact of two focal metastatic tumors (illustrated in red; see also cryosection blockface, (top)) on the ventral callosal boundary, as well as the parieto-occipital and anterior and posterior calcarine sulci in both brain hemispheres. |
Pathology Detection. To identify differences in brain structure between two groups, or between an individual subject and a database of demographically matched subjects, we define Wij(x) as the deformation vector required to match the structure at position x in an atlas template with its counterpart in subject i of group j, and model the deformations as:
Wij(x) = µ j(x) + S (x)1/2e ij(x).
Here µ j(x) is the mean deformation for group j, and S (x) is a non-stationary, anisotropic covariance tensor field, which relaxes the confidence threshold for detecting abnormal structure in regions where normal variability is extreme, S (x)1/2 is the upper triangular Cholesky factor tensor field, and e ij(x) is a trivariate random vector field whose components are independent stationary Gaussian random fields.
A T2 or F statistic which indicates evidence of significant difference in deformations between the groups is calculated at each lattice location in a 3D image or parameterized 3D surface, to form a statistic image (Thompson et al., 1997). Specialized algorithms, using corrections for the metric tensor of the underlying surface, are required to calculate these fields at the cortex (Thompson and Toga, 1998). Under the null hypothesis of no abnormal deformations, the statistic image is approximated by a T2 random field. The global maximum of the random deformation field, or derived tensor fields (Thompson et al., 1998), can be used to test the hypothesis of no structural change in disease (Worsley, 1994a,b; Cao and Worsley, 1998). Similar multivariate linear models can be used to test for the effect of explanatory variables (e.g., age, gender, clinical test scores) on a set of deformation field images.
Probabilistic atlases based on random deformation fields, and associated scalar fields derived using operators which emphasize specific deformational characteristics, have been used to assess gender-specific differences in the brain (Davatzikos, 1996; Cao and Worsley, 1998). These algorithms are currently being tested on databases of 3D MRI and high-resolution cryosection volumes with the goal of detecting structural abnormalities in schizophrenia (Moussai et al., 1998), and in neurodegenerative disorders such as Alzheimer's disease (Figs. 4-6; Thompson et al., 1997, 1998; Mega et al., 1998).
In summary, group-specific patterns of brain structure may go unnoticed in individual subjects or groups due to extreme variations in anatomy between subjects. Population-based brain atlases, however, linked with appropriate warping algorithms, can incorporate extensive regional information on structural variability, and show great promise in identifying group trends and characteristics, especially in disease states.
X. Conclusion
Extreme variations in callosal anatomy are found in normal and diseased populations. These variations complicate the design of systems to detect callosal anomalies in disease, and to clarify associations between callosal structure and sex, handedness, and a variety of behavioral and cognitive factors.
In this chapter, we have reviewed the perplexing variety of methods available for analyzing callosal structure. In view of the controversy over callosal differences and their determinants, probabilistic reference systems based on large human populations may help to identify group-specific patterns of callosal structure, providing a sample size appropriate to investigate subtle effects. Anatomical models can be combined with anatomically-driven elastic transformations which associate homologous brain regions in a database of anatomic data. These strategies provide the ability to perform morphometric comparisons and correlations in three dimensions between a given subject's MR scan and a population database, or between population sub-groups stratified according to relevant clinical and/or demographic criteria.
In many ways, static representations of brain structure are ill-suited to analyzing the dynamic processes of brain development and disease. The inherently changing morphology complicates attempts to compare callosal anatomy across subjects and groups, and interaction effects between age, sex and other demographic factors are also observed. The intense interest in brain development and disease mandates the design of mathematical systems to track anatomical changes over time, and map dynamic patterns of growth or degeneration. In this chapter, we introduced an approach to map patterns of growth at the callosum, emphasizing the regional complexity of growth patterns over a prolonged period.
In the near future, brain mapping techniques will provide the ability to map growth and degeneration in their full spatial and temporal complexity. In spite of the logistic and technical challenges, these mapping approaches hold tremendous promise for representing, analyzing and understanding the extremely complex dynamic processes that affect regional anatomy in the healthy and diseased brain.
.......................
References
Aboitiz F, Rodriguez E, Olivares R, Zaidel E (1996) Age-related changes in fiber composition of the human corpus callosum: sex differences. NeuroReport, 7: 1761-1764.
Aboitiz F, Scheibel AB, Fisher RS, Zaidel E Fiber composition of the human corpus callosum. Brain Res 1992 Dec 11;598(1-2):143-153.
Aboitiz F, Scheibel AB, Fisher RS, Zaidel E: Individual differences in brain asymmetries and fiber composition in the human corpus callosum. Brain Research 1992;598:154-161.
Allen LS, Gorski RA Sex difference in the bed nucleus of the stria terminalis of the human brain. J Comp Neurol 1990 Dec 22;302(4):697-706
Allen LS, Hines M, Shryne JE, Gorski RA Two sexually dimorphic cell groups in the human brain. J Neurosci 1989 Feb;9(2):497-506.
Allen LS, Richey MF, Chai YM, Gorski RA (1991) Sex differences in the corpus callosum of the living human being. The Journal of Neuroscience, 11(4): 933-942.
Amit Y, Grenander U, Piccioni M (1991). Structural Image Restoration through Deformable Templates, J. Amer. Stat. Assoc., 86(414):376-386.
Andreasen NC, Arndt S, Swayze V, Cizadlo T, Flaum M, O'Leary D, Ehrhardt JC, Yuh WTC: Thalamic abnormalities in schizophrenia visualized through magnetic resonance imaging. Science 1994;266:294-298.
Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, Watkins GL, Hichwa RD: Schizophrenia and cognitive dysmetria: a positron emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proceedings of the National Academy of Sciences USA 1996;93:18:9985-90.
Armstrong E (1990) Evolution of the brain. The human nervous system. Academic Press; San Diego CA.
Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW (1991). The Topographical and Neuroanatomical Distribution of Neurofibrillary Tangles and Neuritic Plaques in the Cerebral Cortex of Patients with Alzheimer's Disease, Cerebral Cortex 1:103-116.
Ashburner J, Neelin P, Collins DL, Evans AC, Friston KJ (1997). Incorporating Prior Knowledge into Image Registration, Neuroimage 6(4):344-352.
Barkovich AJ, Kjos BO (1988) Normal postnatal development of the corpus callosum as demonstrated by MR imaging. American Journal of Neuroradiology, 9: 487-491.
Barkovich AJ, Norman D (1988) Anomalies of the corpus callosum: correlation with further anomalies of the brain. American Journal of Neuroradiology, 9: 493-501.
Barta PE, Powers RE, Aylward EH, Chase GA, Harris GJ, Rabins PV, Tune LE, Pearlson GD: Quantitative MRI changes in late onset schizophrenia and Alzheimer's disease compared to normal controls. Psychiatry Res 1997;68:65-75.
Bartley AJ, Jones DW, Torrey EF, Zigun JR, Weinberger DR: Sylvian fissure asymmetries in monozygotic twins: a test of laterality in schizophrenia. Biol Psychiatry 1993; 34:853-63.
Baumgardner TL, Singer HS, Denckla MD, Rubin BS, Abrams BA, Colli MJ, Reiss AL (1996) Corpus callosum morphology in children with Tourette syndrome and attention deficit hyperactivity disorder. Neurology, 47:477-482.
Beaton AA: The relation of planum temporale asymmetry and morphology of the corpus callosum to handedness, gender and dyslexia: a review of the evidence. Brain and Language 1997;60:255-322.
Beigon A, Eberling JL, Richardson BC, Roos MS, Wong ST, Reed BR, Jagust WJ (1994). Human Corpus Callosum in Aging and Alzheimer's Disease: a Magnetic Resonance Imaging Study, Neurobiol. Aging 15(4):393-397.
Bell AD, Variend S (1985) Failure to demonstrate sexual dimorphism of the corpus callosum in childhood. Journal of Anatomy, 143: 143-147 .
Bigelow LB, Nasrallah HA, Rausher FP: Corpus Callosum thickness in chronic schizophrenia. Arch Gen Psychiatry 1983;142:284-287.
Bishop KM, Wahsten, D: Sex differences in the human corpus callosum: myth of reality? Neuroscience and Biobehavioral Reviews 1997;21:581-601.
Black SE, Szekely C, Szalai JP, Kidron D, Yu D, Parker J, Buck B, Stanchev P, Bronskill M (1996). Can MRI Brain Measures Distinguish Alzheimer's Disease from Normal Aging?, Poster Presentation, 2nd. Int. Conf. on Functional Mapping of the Human Brain, June 17-21, 1996, Boston, MA, NeuroImage 3(3):S476.
Blacker D, Albert MS, Bassett SS, Go RC, Harrell LE, Folstein MF (1994). Reliability and Validity of NINCDS-ADRDA Criteria for Alzheimer's Disease. The National Institute of Mental Health Genetics Initiative, Arch. Neurol. 51(12):1198-1204.
Bleier R (1985) Sex Differences Research in the Neurosciences: Science or Belief? Soc. Neurosci. Address, Dallas, Texas, 1985.
Bogen JE, Fisher ED, Vogel PJ Cerebral commissurotomy. A second case report JAMA 1965 Dec 20;194(12):1328-1329.
Bogerts B: The temporolimbic system theory of positive schizophrenia symptoms. Schizophrenia Bull 1998;23:3:423-434.
Bookstein F (1989). Principal Warps: Thin-Plate Splines and the Decomposition of Deformations, IEEE Trans. Pattern Analysis and Machine Intelligence, 11(6):567-585.
Bookstein FL (1997). Landmark Methods for Forms Without Landmarks: Morphometrics of Group Differences in Outline Shape, Medical Image Analysis 1(3):225-243.
Braak H, Braak E (1991). Neuropathological Staging of Alzheimer-Related Changes, Acta Neuropathol. 82:239-259.
Brown JW, Jaffe J (1975) Hypothesis on cerebral dominance. Neuropsychologia, 13: 107-110.
Brown R, Colter N, Corsellis J, Crow TJ, Frith CD, Jangoe K, Johnston EC, Marsh C: Postmortem evidence of structural brain changes in schizophrenia. Arch Gen Psychiatry 1986; 43, 36-42.
Brun A, Englund E (1981). Regional Pattern of Degeneration in Alzheimer's Disease: Neuronal Loss and Histopathologic Grading, Histopathology 5:549-564.
Buchanan RW, Stevens JR, Carpenter WT: The neuroanatomy of schizophrenia: Editor's introduction. Schizophrenia Bull 1998;23:3:365-366.
Burke HL, Yeo RA (1994). Systematic Variations in Callosal Morphology: The Effects of Age, Gender, Hand Preference, and Anatomic Asymmetry, Neuropsychology 8:563-571.
Byne W, Bleier R, Houston L (1988) Variations in human corpus callosum do not predict gender: a study using magnetic resonance imaging. Behavioral Neuroscience, 102(2): 222-227.
Cao J, Worsley KJ (1998). The Geometry of the Hotelling's T-squared Random Field with Applications to the Detection of Shape Changes, Annals of Statistics, [in press].
Capitani E, Della Sala S, Spinnler H (1990). Controversial Neuropsychological Issues in Alzheimer's Disease: Influence of Onset-Age and Hemispheric Asymmetry of Impairment, Cortex 26(1):133-145.
Carlson M, Earls F, Todd RD (1988) The importance of regressive changes in the development of the nervous system: towards a neurobiological theory of child development. Psychiatric Development, 1: 1-22.
Carpenter WT, Buchanan RW, Kirkpatrick B, Tamminga C, Wood F: Strong inference, theory testing, and the neuroanatomy of schizophrenia. Arch of Gen Psychiatry 1993;50:825-831.
Cassanova MF, Sanders RD, Goldberg TE, Bigelow LB, Christison G, Torrey EF, Weinberger DR: Morphometry of the corpus callosum in monozygotic twins discordant for schizophrenia: a magnetic resonance imaging study. J Neurology, Neurosurgery and Psychiatry 1990;53:416-21.
Christensen GE, Rabbitt RD, Miller MI (1993). A Deformable Neuroanatomy Textbook based on Viscous Fluid Mechanics, 27th Ann. Conf. on Inf. Sciences and Systems, 211-216.
Christensen GE, Rabbitt RD, Miller MI (1996). Deformable Templates using Large Deformation Kinematics, IEEE Trans. on Image Processing, Oct. 1996, 5(10):1435-1447.
Chugani HT, Phelps ME, Mazziotta JC (1987) Positron emission tomography study of human brain functional development. Annals of Neurology, 22: 487-497.
Clarke JM, Zaidel E: Anatomical-behavioral relationships: corpus callosum morphometry and hemispheric specialization. Behavioral Brain Research 1994;64:185-202.
Clarke S, Kraftsik R, Van der Loos H, Innocenti GM (1989) Forms and measures of adult and developing human corpus callosum. Journal of Neuropathology and Experimental Neurology, 280: 213-230.
Coffey CE, Wilkinson WE, Parashos IA, Soady SA, Sullivan RJ, Patterson LJ, Figiel GS, Webb MC, Spritzer CE, Djang WT (1992) Quantitative cerebral anatomy of the aging human brain: a cross-sectional study using magnetic resonance imaging. Neurology, 42(3)1:527-36.
Coger RW, Serafetinides EA: Schizophrenia, corpus callosum, and interhemispheric transfer: a review. Psychiatry Res 1990;34:163-84.
Cole M, Cole SR (1993) The Development of Children. Scientific American Books, New York, NY.
Coleman PD, Flood DG (1987). Neuron Numbers and Dendritic Extent in Normal Aging and Alzheimer's Disease, Neurobiol. Aging 8:521-545.
Colombo C, Bonfanti A, Livian S, Abbruzzese M, Scarone S: Size of the corpus callosum and auditory comprehension in schizophrenics and normal controls. Schizophrenia Research 1993;11:1:63-70.
Corder EH, Jelic V, Basun H, Lannfelt L, Valind S, Winblad B, Nordberg A (1997). No Difference in Cerebral Glucose Metabolism in Patients with Alzheimer Disease and Differing Apolipoprotein E Genotypes, Arch. Neurol. 54(3):273-277.
Crow TJ: Temporolimbic or transcallosal connections: where is the primary lesion in schizophrenia and what is its nature. Schizophrenia Bull 1998;23:3:521-523.
Cuénod CA, Denys A, Michot JL, Jehenson P, Forette F, Kaplan D, Syrota A, Boller F (1993). Amygdala Atrophy in Alzheimer's Disease: An In Vivo Magnetic Resonance Imaging Study, Arch. Neurol. 50:941-945.
Cunningham DJ (1892). Contribution to the Surface Anatomy of the Cerebral Hemispheres, Cunningham Memoirs (R. Irish Acad.) 7:372.
Davatzikos C (1996a). Spatial Normalization of 3D Brain Images using Deformable Models, J. Comp. Assisted Tomography 20(4):656-665, Jul.-Aug. 1996.
Davatzikos C, Prince JL (1996). Convexity Analysis of Active Contour Problems, Proceedings of CVPR, San Francisco, June 17-20, 1996.
Davatzikos C, Vaillant M, Resnick SM, Prince JL, Letovsky S, Bryan RN (1996b). A Computerized Approach for Morphological Analysis of the Corpus Callosum, J. Comp. Assisted Tomography, 20(1):88-97.
David AS, Minne C, Jones P, Harvey I, Ron MA: Structure and function of the corpus callosum in schizophrenia: what's the connection? Eur. Psychiatry 1995;10:28-35.
David AS: Tachistoscopic tests of colour naming and matching in schizophrenia: evidence for posterior callosum dysfunction? Psychological Medicine 1987;17:3:621-30.
David AS: Schizophrenia and the corpus callosum: developmental, structural and functional relationships. Behavioral Brain Res 1994;64:203-211.
Davidson RJ, Hugdahl K (1994). Brain Asymmetry, MIT Press.
Davis PC, Mirra SS, Alazraki N (1994). The Brain in Older Persons with and without Dementia: Findings on MR, PET and SPECT Images (Review Article), Amer. J. Radiol., 162:1267-1278.
DeCarli C, Grady CL, Clark CM, Katz DA, Brady DR, Murphy DG, Haxby JV, Salerno JA, Gillette JA, Gonzalez-Aviles A, Rapoport SI (1996). Comparison of Positron Emission Tomography, Cognition, and Brain Volume in Alzheimer's Disease with and Without Severe Abnormalities of White Matter, J. Neurol. Neurosurg. Psychiatry 60(2):158-167.
Degreef G, Bogerts B, Falkai P, Greve B, Lantos G, Ashtari M, Leiberman J: Increased prevalence of the cavum septum pellucidum in magnetic resonance scans and post-mortem brains of schizophrenic patients. Psychiatry Res 1992;45;1-13.
DeLacoste MC, Kirkpatrick JB, Ross ED (1985) Topography of the human corpus callosum. Journal of Neuropathology and Experimental Neurology, 44(6): 578-591.
DeLacoste-Utamsing MC, Holloway RL: Sexual dimorphism in the human corpus callosum. Science 1982;216:1431-1432.
Delaère P, Duyckaerts C, Brion JP, Poulain V, Hauw JJ (1989). Tau, Paired Helical Filaments and Amyloid in the Neocortex: A Morphometric Study of 15 Cases with Graded Intellectual Status in Aging and Senile Dementia of Alzheimer Type, Acta Neuropathologica (Berlin) 77(6):645-653.
DeLisi LE, Dauphinais ID, Hauser P: Gender differences in the brain: are they relevant to the pathogenesis of schizophrenia? Compar Psychiatry 1989;30:197-208.
DeLisi LE, Hoff AL, Neale C, Kushner M: Asymmetries in the superior temporal lobe in male and female first-episode schizophrenic patients: measures of the planum temporale and superior temporal gyrus by MRI. Schizophrenia Res 1994;12:19-28.
DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R: Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res 1997;74:3:129-40.
DeLisi LE, Tew W, Xie S, Hoff AL, Sakuma M, Kushner M, Lee G, Shedlack K, Smith AM, Grimson R: A prospective follow-up study of brain morphology and cognition in first-episode schizophrenic patients: preliminary findings. Biol Psychiatry 1995;38:349-360.
Demeter S, Rongo JL, Doty RW (1985). Sexual Dimorphism of the Human Corpus Callosum? Soc. Neurosci. Abstr. 11:868.
Denenberg VH, Kertesz A, Cowell PE A factor analysis of the human's corpus callosum. Brain Res 1991 May 10;548(1-2):126-132.
DeQuardo JR, Bookstein FL, Green WD, Brunberg JA, Tandon R: Spatial relationships of neuroanatomic landmarks in schizophrenia. Psychiatry Res 1996;61:81-95.
Di Patre PL (1990). Cytoskeletal Alterations Might Account for the Phylogenetic Vulnerability of the Human Brain to Alzheimer's Disease, Medical Hypotheses 34:165-170.
Diamond A (1990) The development and neural basis of higher cognitive functions. Annals of the New York Academy of Sciences, 608: 267-317.
Ditchfield H, Hemsley DR: Interhemispheric transfer of information and schizophrenia. European Arch Psychiatry and Neurol Sciences. 1990:239;309-13.
DiVirgilio G, Clarke S. Direct interhemispheric visual input to human speech areas. Human Brain Mapping, 1997, 5(5):347-354.
Doraiswamy PM, Figiel GS, Husain MM, McDonald WM, Shah SA, Boyko OB, Ellinwood EH, Krishnan KRR (1991) Aging of the human corpus callosum: magnetic resonance in normal volunteers. J. Neuropsychiatry and Clinical Neurosciences, 3: 392-397.
Duara R, Kushch A, Gross-Glenn K, Barker WW, Jallad B, Pascal S, Loewenstein DA, Sheldon J, Rabin M, Levin B (1991). Neuroanatomic Differences between Dyslexic and Normal Readers on Magnetic Resonance Imaging Scans, Arch. Neurol. 48:410-416.
Eberstaller O (1884). Zür Oberflachen Anatomie der Grosshirn Hemisphaeren, Wien Med. Bl., 7:479,642,644.
Eidelberg D, Galaburda AM (1982). Symmetry and Asymmetry in the Human Posterior Thalamus: I. Cytoarchitectonic Analysis in Normal Persons, Arch. Neurol. 39(6):325-332.
Erkinjuntti T, Lee DH, Gao F, Steenhuis R, Eliasziw M, Fry R, Merskey H, Hachinski VC (1993). Temporal Lobe Atrophy on Magnetic Resonance Imaging in the Diagnosis of Early Alzheimer's Disease, Arch. Neurol. 50(3):305-310.
Evans AC, Dai W, Collins DL, Neelin P, Marrett S (1991). Warping of a Computerized 3D Atlas to Match Brain Image Volumes for Quantitative Neuroanatomical and Functional Analysis, SPIE Med. Imaging 1445:236-247.
Feng JZ, Brugge JF (1983) Postnatal development of auditory callosal connections in the kitten. Journal of Comparative Neurology, 214: 416-426.
Ferrario VF, Sforza C, Serrao G, Frattini T, del Favero C (1996) Shape of the human corpus callosum in childhood: elliptic fourier analysis on midsagittal magnetic resonance scans. Investigative Radiology, 31(1): 1-5.
Flaum M, Swayze VW, O'Leary DS, Yuh WTC, Ehrhardt JC, Arndt SV, Andreasen NC. Effects of diagnosis, laterality, and gender on brain morphology in schizophrenia. Am J of Psychiatry 1995;152:5704-714.
Frangou S, Sharma t, Sigmudsson T, Barta P, Pearlson G, Murray RM: The Maudsley Family study.4: Normal planum temporale asymmetry in familial schizophrenia: a volumetric MRI study. Br J of Psychiatry 1997;170:230-33.
Freeborough PA, Woods RP, Fox NC (1996). Accurate Registration of Serial 3D MR Brain Images and its Application to Visualizing Change in Neurodegenerative Disorders, J. Comput. Assist. Tomogr. 20(6):1012-1022, Nov. 1996.
Freedman M, Albert ML (1985) Subcortical Dementia. In: Fredericks JAM [ed.], Handbook of Clinical Neurology: Neurobehavioral Disorders, vol. 46. Elsevier, Amsterdam, 311-316.
Friedland RP, Luxenberg J (1988). Neuroimaging and Dementia, in: Clinical Neuroimaging: Frontiers in Clinical Neuroscience, vol. 4, Theodore WH [ed.], Allan Liss, Inc., New York, 139-163.
Gaffney GR, Kuperman S, Tsai LY, Minchin S, Hassanein KM (1987) Midsagittal magnetic resonance imaging of autism. British Journal of Psychiatry, 151: 831-833.
Galaburda AM (1995). Anatomic Basis of Cerebral Dominance, in: Brain Asymmetry, Davidson RJ, Hugdahl K [eds.], Boston, MIT Press, 51-73.
Galaburda AM, Geschwind N (1981). Anatomical Asymmetries in the Adult and Developing Brain and their Implications for Function, Adv. Pediatr. 28:271-292.
Galaburda AM, Rosen GD, Sherman GF: Individual variability in cortical organization: its relationship to brain laterality and implications to function, Neuropsychologica 1990;28:529-546.
Gazzaniga MS: Principles of human brain organization derived from split-brain studies. Neuron 1995;14:217-28.
Gee JC, Reivich M., Bajcsy R (1993). Elastically Deforming an Atlas to Match Anatomical Brain Images, J. Comp. Assist. Tomogr. 17(2):225-236, March 1993.
Georgy BA, Hesselink JR, Jernigan TL (1993) MR imaging of the corpus callosum. American Journal of Radiology, 160: 949-955.
Geschwind N, Galaburda AM, Cerebral lateralization. Biological mechanisms, associations and pathology. Arch Neurology 1985;428-459.
Geschwind N, Levitsky W (1968). Human Brain: Left-Right Asymmetries in Temporal Speech Region, Science 161: 186.
Giedd JN, Castellanos FX, Casey BJ, Kozuch P, King AC, Hamburger SD, Rapaport JL (1994) Quantitative morphology of the corpus callosum in attention deficit hyperactivity disorder. American Journal of Psychiatry, 151: 665-669.
Giedd JN, Rumsey JM, Castellanos FX, Rajapakse JC, Kaysen D, Vaituzis AC, Vauss AC, Hamburger SD, Rapoport JL (1996) A quantitative MRI study of the corpus callosum in children and adolescents. Developmental Brain Research, 91: 274-280.
Goldman-Rakic PS, Selemon LD: Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophrenia Bull 1998;23:3:437-458.
Gómez-Isla T, Price JL, McKeel DW, Morris JC, Growdon JH, Hyman B (1996). Profound Loss of Layer II Entorhinal Cortex Neurons Occurs in Very Mild Alzheimer's Disease, J. Neurosci. 16(14):4491-4500.
Grenander U (1976). Pattern Synthesis: Lectures in Pattern Theory, Applied Math. Sci. 13, Springer-Verlag.
Grenander U, Miller MI (1994). Representations of Knowledge in Complex Systems, J. Royal Statistical Society B, 56(4):549-603.
Gunther W, Petsch R, Steinberg R, Moser E, Streck P, Heller H, Kurtz G, Hippius H: Brain dysfunction during motor activation and corpus callosum alterations in schizophrenia measured by cerebral blood flow and magnetic resonance imaging. Biol Psychiatry. 1991:29;535-55.
Haller JW, Banerjee A, Christensen GE, Gado M, Joshi S, Miller MI, Sheline Y, Vannier MW, Csernansky JG (1997). Three-Dimensional Hippocampal MR Morphometry with High-Dimensional Transformation of a Neuroanatomic Atlas, Radiology, Feb. 1997, 202(2):504-510.
Hauser P, Dauphinais ID, Berrettini W, DeLisi LE, Gelernter J, Post RM: Corpus callosum dimensions measured by magnetic resonance imaging in bipolar affective disorder and schizophrenia. Biological Psychiatry 1989; 26:659-68.
Heidrich A, Strik WK: Auditory P300 topography and neuropsychological test performance: evidence for left hemispheric dysfunction in schizophrenia. Biol Psychiatry 1997;41:327-35.
Hoff AL, Roidan H, O'Donnell D, Stritzke P, Neale C, Boccio A, Anand AK, DeLisi LE: Anomalous lateral sulcus asymmetry and cognitive function in first-episode schizophrenia. Schizophrenia Bull 1992;18:2:257-270.
Hofmann E, Becker T, Meixensberger J, Jackel M, Schneider M, Reichmann H (1995). Disturbances of Cerebrospinal Fluid (CSF) Circulation--Neuropsychiatric Symptoms and Neuroradiological Contribution, J. Neural Transm. Gen. Sect. 99(1-3):79-88.
Holloway RL, de Lacoste MC: Sexual dimorphism in the corpus callosum: An extension and replication study. Human Neurobiol 1986;5:87-91.
Horrobin DF, Glen AI, Hudson CJ: Possible relevance of phospholipid abnormalities and genetic interactions in psychiatric disorders: the relationship between dyslexia and schizophrenia. Med Hypotheses 1995; 45:605-13.
Huttenlocher PR (1990) Morphometric study of human cerebral cortex development. Neuropsychologia, 28: 517-527.
Hyde T, Stacey M, Coppola R, Handel S, Rickler K, Weinberger D (1995) Cerebral morphometric abnormalities in Tourette's syndrome: a quantitative MRI study of monozygotic twins. Neurology, 45: 1176-1182.
Hynd GW, Hall J, Novey ES, Eliopulos RT, Black K, Gonzalez JJ, Edmonds JE, Riccio C, Cohen M (1995) Dyslexia and corpus callosum morphology, 52: 32-38.
Hynd GW, Semrud-Clikeman M, Lorys AR, Novey ES, Eliopulos D, Lyytinen J (1991) Corpus callosum morphology in attention deficit hyperactivity disorder: morphometric analysis of MRI. Journal of Learning Disabilities, 24:141-146.
Innocenti GM (1994) Some new trends in the study of the corpus callosum. Behavioural Brain Research, 64: 1-8.
Ishii N, Nishihara Y, Imamura T (1986). Why Do Frontal Lobe Symptoms Predominate in Vascular Dementia with Lacunes? Neurology 36:340-345.
Ivy GO, Killackey H (1981) The ontogeny of the distribution of callosal projection neurons in rat parietal cortex. Comparative Neurology, 195: 367-389.
Jacobsen LK, Geidd JN, Rajapakse JC, Hamberger SD, Vaituzis AC, Frazier JA, Lenane MC, Rapoport JL: Quantitative magnetic resonance imaging of the corpus callosum in childhood onset schizophrenia. Psychiatry Res 1997:68;77-86.
Jäncke L, Staiger JF, Schlaug G, Huang Y, Steinmetz H: The relationship between corpus callosum size and forebrain volume. Cerebral Cortex 1997:7;1047-3211.
Janowsky JS, Kaye JA, Carper RA (1996). Atrophy Of The Corpus Callosum In Alzheimer's Disease Versus Healthy Aging, J. Am. Geriatr. Soc. 44:798-803.
Jernigan TL, Bellugi U, Sowell E, Doherty S, Hesselink JR (1993) Cerebral morphological distinctions between Williams and Down Syndromes. Archives of Neurology, 50:186-191.
Jernigan TL, Salmon DP, Butters N, Hesselink JR (1991). Cerebral Structure on MRI: Part I. Localization of Age-Related Changes, Biol. Psychiatry 29:55-67.
Johnson VP, Swayze VW, Sato Y, Andreason NC (1996) Fetal alcohol syndrome: craniofacial and central nervous system manifestations. American Journal of Medical Genetics, 61:329-339.
Joshi S, Miller MI, Grenander U (1998). On the Geometry and Shape of Brain Sub-Manifolds, Int. J. Patt. Recogn. and Artif. Intell. [submitted].
Katzman R (1986). Alzheimer's Disease, New Engl. J. Med. 314:964.
Kaufer DI, Miller BL, Itti L, Fairbanks LA, Li J, Fishman J, Kushi J, Cummings JL (1997). Midline Cerebral Morphometry Distinguishes Frontotemporal Dementia and Alzheimer's Disease, Neurology 48(4):978-985.
Khachaturian ZS (1985). Diagnosis of Alzheimer's Disease, Arch. Neurol. 42: 1097-1105.
Kido DK, Caine ED, LeMay M, Ekholm S, Booth H, Panzer R (1989). Temporal Lobe Atrophy in Patients with Alzheimer Disease: A CT Study, AJNR 7:551-555.
Kier EL, Truwit CL (1996) The normal and abnormal genu of the corpus callosum: an evolutionary, embryologic, anatomic and MR analysis. American Journal of Neuroradiology, 17: 1631-1641.
Kikinis R, Shenton ME, Gerig G, Hokama H, Haimson J, O'Donnell BF, Wible CG, McCarley RW, Jolesz FA: Temporal lobe sulco-gyral pattern anomalies in schizophrenia: an in vivo MR three-dimensional surface rendering study. Neuroscience Letters 1994;182:7-12.
Killiany RJ, Moss MB, Albert MS, Sandor T, Tieman J, Jolesz F (1993). Temporal Lobe Regions on Magnetic Resonance Imaging Identify Patients with Early Alzheimer's Disease, Arch. Neurol. 50:949-954.
Kleinschmidt A, Falkai P, Huang Y, Schneider T, Furst G, Steinmetz H: In vivo morphometry of planum temporale asymmetry in first-episode schizophrenia. Schizophrenia Res 1994;12:9-18.
Laissy JP, Patrux B, Duchateau C, Hannequin D, Hugonet P, Ait-Yahia H, Thiebot J (1993) Midsagittal MR measurements of the corpus callosum in healthy subjects and diseased patients: a prospective study. American Journal of Neuroradiology, 14: 145-154.
LaMantia AS, Rakic P (1990). Axon Overproduction and Elimination in the Corpus Callosum of the Developing Rhesus Monkey, J. Neurosci. 10(7):2156-2175.
Larsen JP, Høien T, Ödegaard H (1992). Magnetic Resonance Imaging of the Corpus Callosum in Developmental Dyslexia, Cognitive Neuropsychology 9:123-134.
Lawrie SM, Abukmeil SS: Brain abnormality in schizophrenia. Br J of Psychiatry 1998;172:110-120.
Lim KO, Sullivan EV, Zipursky RB, Pfefferbaum A: Cortical gray matter volume deficits in schizophrenia: a replication. Schizophrenia Research 1996;20:157-164.
Loewenstein DA, Barker WW, Chang JY, Apicella A, Yoshii F, Kothari P, Levin B, Duara R (1989). Predominant Left Hemisphere Metabolic Dysfunction in Dementia, Arch. Neurol. 46(2):146-152.
Lyoo IK, Noam GG, Chang KL, Ho KL, Kennedy BP, Renshaw PF (1996) The corpus callosum and lateral ventricles in children with attention deficit hyperactivity disorder: a brain magnetic resonance imaging study. Biological Psychiatry, 40:1060-1063.
Lyoo IK, Satlin A, Lee CK, Renshaw PF Regional atrophy of the corpus callosum in subjects with Alzheimer's disease and multi-infarct dementia. Psychiatry Res 1997 May 16;74(2):63-72.
Marsh L, Harris D, Lim KL, Beal M, Hoff AL, Minn K, Csernansky JG, DeMent S, Faustman WO, Sullivan EV, Pfefferbaum A: Structural magnetic resonance imaging abnormalities in men with severe chronic schizophrenia and an early are at clinical onset. Arch Gen Psychiatry 1997;54:1104-1112.
Mazziotta JC, Toga AW, Evans AC, Fox P, Lancaster J (1995) A Probabilistic Atlas of the Human Brain: Theory and Rationale for its Development , NeuroImage 2: 89-101.
McKeever WF, Jackson TL Jr: Cerebral dominance assessed by object- and color-naming latencies: sex and familial sinistrality effects. Brain and Language 1979;7:2:175-90.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadian EM (1984). Clinical Diagnosis of Alzheimer's Disease: Report of the NINCDS-ARDRA Work Group under the Auspices of the Health and Human Services Task Force on Alzheimer's Disease, Neurology 34:939-944.
Mega MS, Thompson PM, Cummings JL, Back CL, Xu LQ, Zohoori S, Goldkorn A, Moussai J, Fairbanks L, Small GW, Toga AW (1998) Sulcal Variability in the Alzheimer's Brain: Correlations with Cognition, Neurology 50:145-151, January 1998.
Menon RR, Barta PE, Aylward EH, Richards SS, Vaughn DD, Tien AY, Harris GJ, Pearlson GD: Posterior superior temporal gyrus in schizophrenia: grey matter changes and clinical correlates. Schizophrenia Research 1995;16:127-35.
Moussai J, Anvar BA, Narr KL, Cannestra AF, Thompson PM, Sharma T, Toga AW (1998). 3-Dimensional Analysis of Lateral Ventricles in Schizophrenia, 5th Int. Conf. on Human Brain Mapping, [in press].
Murphy DGM, DeCarli CD, Daly E, Gillette JA, McIntosh AR, Haxby JV, Teichberg D, Schapiro MB, Rapoport SI, Horwitz B (1993). Volumetric Magnetic Resonance Imaging in Men with Dementia of the Alzheimer Type: Correlations with Disease Severity, J. Biological Psychiatry 34:612-621.
Nasrallah HA: The unintergrated right cerebral hemispheric consciousness as alien intruder. Comprehensive Psychiatry 1985;26:273-282.
Nestor PG, Shenton ME, McCarley RW, Haimson J, Smith RS, O'Donnell B, Kimble M, Kikinis R, Jolesz FA: Neuropsychological correlates of MRI temporal lobe abnormalities in schizophrenia. Am J of Psychiatry 1993;150:12:1849-55.
Njiokiktjien C, de Sonneville L, Vaal J (1991) Callosal size in children with learning disabilities. Behavioral Brian Research, 64: 213-218
Nopoulos P, Swayze V, Andreasen NC: Patterns of brain morphology in patients with schizophrenia and large cavum septi pellucidi. J of Neuropsychiatry and Clinical Neurosciences 1996;8(2)147-52.
Nopoulos P, Torres I, Flaum M, Andreasen NC, Ehrhardt JC, Yuh WTC: Brain morphology in first-episode schizophrenia. Am J of Psychiatry 1995;152:1721-1723.
Oppenheim JS, Skerry JE, Tramo MJ, Gazzaniga MS Magnetic resonance imaging morphology of the corpus callosum in monozygotic twins. Ann Neurol 1989 Jul;26(1):100-104.
Pakkenberg H, Voigt H (1964). Brain Weight of the Danes, Acta Anat. 56:297-307.
Pandya DN, Seltzer B: The topography of commisural fibers. In F Lepore, M Ptito, HH Jasper (Eds.), Two hemispheres - one brain: functions of the corpus callosum, Alan R Liss, New York 1986 pp. 47-73.
Parashos IA, Wilkinson WE, Coffey CE (1993) Magnetic resonance imaging of the corpus callosum: predictors of size in normal adults. Journal of Neuropsychiatry and Clinical Neurosciences, 7: 35-41.
Pearson RC, Esiri MM, Hiorns RW, Wilcock GK, Powell TP (1985). Anatomical Correlates of the Distribution of the Pathological Changes in the Neocortex in Alzheimer Disease, Proc. Natl. Acad. Sci. USA 82(13):4531-4534.
Peterson BS, Leckman JF, Duncan JS (1994) Corpus callsoum morphology from magnetic resonance images in Tourette's syndrome. Psychiatry Research in Neuroimaging, 55:85-99.
Petty RG, Barta PE, Pearlson GD, McGilchrist IK, Lewis RW, Tien AY, Pulver A, Vaughn DD, Casanova MF, Powers RE: Reversal of asymmetry of the planum temporale in schizophrenia. Am J of Psychiatry 1995;152:5:715-721.
Pilowsky LS, Kerwin RW, Murray RM: Schizophrenia: a neurodevelopmental perspective. Neuropsychopharmacology 1993;9:83-91.
Piven J, Bailey J, Ranson BJ, Arndt S (1997) An MRI study of the corpus callosum in autism. American Journal of Psychiatry, 154(8): 1051-1056.
Pozzilli C, Bastianello S, Padovani A, Passafiume D, Millefiorini E, Bozzao L, Fieschi C (1991). Anterior Corpus Callosum Atrophy and Verbal Fluency in Multiple Sclerosis, Cortex 27(3):441-445.
Pujol J, Vendrell P, Junque C, Marti-Vilalta JL, Capdevila A (1992) When does human brain development end? Evidence of corpus callosum growth up to adulthood. Annals of Neurology, 34(1): 71-74.
Raine A, Harrison GN, Reynolds GP, Sheard C, Cooper JE, Meddley I: Structural and functional characteristics of the corpus callosum in schizophrenics, psychiatric controls, and in normals. Arch of Gen Psychiatry 1990;471060-1064..
Rajapakse JC, Giedd JN, Rumsey JM, Vaituzis AC, Hamburger SD, Rapoport JL (1996) Regional MRI measurements of the corpus callosum: a methodological and developmental study. Brain and Development, 18: 379-388.
Rakic P, Yakovlev PI (1968) Development of the corpus callosum and cavum septi in man. Journal of Comparative Neurology, 26: 100-104.
Randall PL: Schizophrenia, abnormal connection and brain evolution. Medical Hypotheses 1983;10:247-280.
Rauch RA, Jinkins JR (1994) Analysis of cross-sectional area measurements of the corpus callosum adjusted for brain size in male and female subjects from childhood to adulthood. Behavioural Brain Research, 64: 65-78.
Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL (1995) Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcoholism: Clinical and Experimental Research, 19(5): 1198-1202.
Rizzo G, Gilardi MC, Prinster A, Grassi F, Scotti G, Cerutti S, Fazio F (1995). An Elastic Computerized Brain Atlas for the Analysis of Clinical PET/SPET Data, Eur. J. Nucl. Med. 22(11):1313-18.
Roa SM, Bernadin L, Leo GL, et al (1989) Cerebral disconnection in multiple sclerosis: relationship to atrophy in the corpus callosum. Archives of Neurology, 46, 918-920.
Roa SM, Leo GL, Haughton VM et al (1989) Correlation of MRI with neuropsychological testing in multiple sclerosis. Neurology, 39:161-166.
Rossi A, Stratta P, Gallucci M, Passariello R, Casacchia M: Quantification of corpus callosum and ventricles in schizophrenia with nuclear magnetic resonance imaging: a pilot study. Am J Psychiatry 1989;146:99-101.
Rumsey JM, Andreason P, Zametkin AJ, Aquino T, King AC, Hamburger SD, Pikus A, Rapoport JL, Cohen RM (1994) Failure to activate the left temporoparietal cortex in dyslexia. An oxygen-15 positron emission tomographic study, Neurology, 49:527-534.
Saitoh O, Courchesne E, Egaas B, Loncoln AJ, Schreibman L (1995) Cross-sectional area of the psoterior hippocampus in autistic patients with cerebellar and corpus callosum abnormalities. Neurology, 45: 317-323.
Salat D, Ward A, Kaye JA, Janowsky JS (1997) Sex differences in the corpus callosum with aging. Neurobiology of Aging, 18(2): 191-197.
Sandor SR, Leahy RM (1995). Towards Automated Labeling of the Cerebral Cortex using a Deformable Atlas, In: Bizais Y, Barillot C, Di Paola R [eds.], Info. Proc. in Med. Imag., June 1995, 127-138.
Schaefer GB, Thompson JN, Bodensteiner JB, Hamza M, Tucker RR, Marks W, Gay C, Wilson D (1990) Quantitative morphometric analysis of brain growth using magnetic resonance imaging. Journal of Child Neurology, 5: 127-130.
Schlaug G, Jancke L, Huang Y, Staiger JF, Steinmetz H Increased corpus callosum size in musicians. Neuropsychologia 1995 Aug;33(8):1047-1055.
Schrift MJ, Bandla H, Shah P, Taylor MA: Interhemispheric transfer in major psychoses.J Nervous and Mental Diseases. 1986:174;203-7.
Schroder J, Buchsbaum MS, Siegel BV, Geider FJ, Neithammer R: Structural and functional correlates of subsyndromes in chronic schizophrenia. Psychopathology 1995:28;38-45.
Scott SA (1993). Dendritic Atrophy and Remodeling of Amygdaloid Neurons in Alzheimer's Disease, Dementia 4(5):264-272.
Semrud-Clikeman M, Filipek PA, Biederman J, Steingard R, Kennedy D, Renshaw P, Bekken K (1994) Attention-deficit hyperactivity disorder: magnetic resonance imaging morphometric analysis of the corpus callosum. J. American Academy of Child and Adolescent Psychiatry, 33(6):875-881.
Shenton ME, Kikinis R, Jolesz FA, Pollack SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley R: Abnormalities of the left temporal lobe and thought disorder in schizophrenia. New England J of Med 1992;327:9:604-612.
Siegel BV Jr, Shihabuddin L, Buchsbaum MS, Starr A, Haier RJ, Valladares Neto DC (1996). Gender Differences in Cortical Glucose Metabolism in Alzheimer's Disease and Normal Aging, J. Neuropsychiatry Clin. Neurosci. 8(2):211-214.
Small GW, Mazziotta JC, Collins MT, Baxter LR, Phelps ME, Mandelkern MA, Kaplan A, La Rue A, Adamson CF, Chang L, et al. (1995). Apolipoprotein E Type 4 Allele and Cerebral Glucose Metabolism in Relatives At Risk for Familial Alzheimer Disease, JAMA 273(12):942-947.
Smith RC, Baumgartner R, Calderon M: Magnetic resonance imaging studies of the brains of schizophrenic patients. Psychiatry Res 1987;20:33-46.
Sobire G, Goutieres F, Tardieu M, Landrieu P, Aicardi J (1995). Extensive Macrogyri or No Visible Gyri: Distinct Clinical, Electroencephalographic, and Genetic Features According to Different Imaging Patterns, Neurology 45:1105-1111.
Staib LH, Duncan JS (1992). Boundary Finding with Parametrically Deformable Models, IEEE Pattern Analysis and Machine Intelligence, Nov. 1992, 14(11):1061-1075.
Steere JC, Arnsten AFT (1995) Corpus callosum morphology in ADHD. American Journal of Psychiatry, 152(7):1105.
Stein JF: Developmental dyslexia, neural timing and hemispheric lateralization. Int J of Psychophysiology 1994; 18:241-9.
Steinmetz H, Staiger JF, Schlaug G, Huang Y, Jancke L: Corpus callosum and brain volume in women and men. Neuroreport 1995;6:7:1002-4.
Stevens JR: Anatomy of schizophrenia revisited. Schizophrenia Bull 1998;23:3:373-383.
Stievenart JL, Iba-Zizen MT, Tourbah A, Lopez A, Thibierge M, Abanou A, Cabanis EA Minimal surface: a useful paradigm to describe the deeper part of the corpus callosum? Brain Res Bull 1997;44(2):117-124.
Strauss E, Kosaka B, Wada J (1983). The Neurobiological Basis of Lateralized Cerebral Function. A Review, Hum. Neurobiol. 2(3):115-127.
Strauss E, Wada J, Hunter M Callosal morphology and performance on intelligence tests. J Clin Exp Neuropsychol 1994 Feb;16(1):79-83.
Sullivan EV, Shear PK, Kelvin OL, Zipursky RB, Pfefferbaum A: Cognitive and motor impairments are related to gray matter volume deficits in schizophrenia. Biol Psychiatry 1996;39:234-240.
Swayze VW, Andreasen NC, Ehrhardt JC, Yuh WT, Alliger RJ, Cohen GA: Developmental abnormalities of the corpus callosum in schizophrenia. Arch of Neurology 1990;47:7:805-8.
Swayze VW, Johnson VP, Hanson JW, Piven J, Sato Y, Giedd JN, Mosnik D, Andreasen NC (1997) Magnetic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatrics, 99(2):232-40.
Talairach J, Szikla G, Tournoux P, Prosalentis A, Bordas-Ferrier M, Covello L, Iacob M, Memphel E: Atlas d'anatomie stereotaxique du telencephale 1967; Paris:Masson.
Talairach J, Tournoux P (1988). Co-planar Stereotaxic Atlas of the Human Brain, New York: Thieme.
Terry RD, de Theresa R, Hansen LA (1987) Neocortical cell counts in normal human adult aging. Annals of Neurology, 21: 530-539.
Thirion J-P, Calmon G (1997) Deformation Analysis to Detect and Quantify Active Lesions in 3D Medical Image Sequences, INRIA Technical Report 3101, Feb. 1997.
Thirion J-P, Prima S, Subsol S (1998). Statistical Analysis of Dissymmetry in Volumetric Medical Images, Medical Image Analysis [in press].
Thompson PM, Giedd JN, Blanton RE, Lindshield C, Woods RP, MacDonald D, Evans AC, Toga AW (1998). Growth Patterns in the Developing Human Brain Detected Using Continuum-Mechanical Tensor Maps and Serial MRI, [in press].
Thompson PM, MacDonald D, Mega MS, Holmes CJ, Evans AC, Toga AW (1997a). Detection and Mapping of Abnormal Brain Structure with a Probabilistic Atlas of Cortical Surfaces, J. Comp. Assist. Tomogr. 21(4):567-581, Jul.-Aug. 1997.
Thompson PM, Moussai J, Khan AA, Zohoori S, Goldkorn A, Mega MS, Small GW, Cummings JL, Toga AW (1998). Cortical Variability and Asymmetry in Normal Aging and Alzheimer's Disease, Cerebral Cortex [in press, Feb. 1998].
Thompson PM, Schwartz C, Lin RT, Khan AA, Toga AW (1996b). 3D Statistical Analysis of Sulcal Variability in the Human Brain, Journal of Neuroscience, Jul. 1996, 16(13):4261-4274.
Thompson PM, Schwartz C, Toga AW (1996a). High-Resolution Random Mesh Algorithms for Creating a Probabilistic 3D Surface Atlas of the Human Brain, NeuroImage 3:19-34.
Thompson PM, Toga AW (1996c). A Surface-Based Technique
for Warping 3-Dimensional Images of the Brain, IEEE Transactions on Medical Imaging, Aug. 1996, 15(4):1-16.Thompson PM, Toga AW (1997b) Detection, Visualization and Animation of Abnormal Anatomic Structure with a Deformable Probabilistic Brain Atlas based on Random Vector Field Transformations, Medical Image Analysis 1(4): 271-294; paper, with video sequences on CD-ROM with Journal Issue, November 1997.
Thompson PM, Toga AW (1998). 3D Brain Image Matching using a Concept from General Relativity, Tech. Report, UCLA Laboratory of Neuro Imaging, Jan. 1998.
Toga AW (1998) Brain Warping, Academic Press.
Toga AW, Thompson PM (1998) An Introduction to Brain Warping, in: Brain Warping, (Toga AW, ed.), Academic Press [in press].
Toga AW, Thompson PM, Payne BA (1996). Modeling Morphometric Changes of the Brain during Development, chapter in: Developmental Neuroimaging: Mapping the Development of Brain and Behavior, Thatcher RW, Lyon GR, Rumsey J, Krasnegor N, eds., Academic Press.
Tomlinson BE, Blessed G, Roth M (1968) Observations of the brains of nondemented old people. Journal of the Neurological Sciences, 7: 331-356.
Uematsu M, Kaiya H: The morphology of the corpus callosum in schizophrenia: an MRI study. Schizophrenia Res 1988;1:391-8.
Vermersch P, Roche J, Hamon M, Daems-Monpeurt C, Pruvo JP, Dewailly P, Petit H (1996). White Matter Magnetic Resonance Imaging Hyperintensity in Alzheimer's Disease: Correlations with Corpus Callosum Atrophy, J. Neurol. 243(3):231-234.
Von Hufsten C (1984) Developmental changes in the organization of prereaching movements. Developmental Psychology, 20: 369-382.
Waddington JL: Neurodynamics of abnormalities in cerebral metabolism and structure in schizophrenia. Schizophrenia Bulletin 1993;19:55-8.
Wahlund LO, Andersson-Lundman G, Basun H, Almkvist O, Bjorksten KS, Saaf J, Wetterberg L (1993). Cognitive Functions and Brain Structures: A Quantitative Study of CSF Volumes on Alzheimer Patients and Healthy Control Subjects, Magn. Reson. Imaging 11(2):169-174.
Waldemar G (1995) Functional brain imaging with SPECT in normal aging and dementia. Methodological, pathophysiological, and diagnostic aspects. Cerebrovascular and Brain Metabolism Reviews, 7(2):89-130.
Wang PP, Doherty S, Hesselink JR, and Bellugi U (1992) Callosal morphology concurs with neurobehavioral and neuropathological findings in two neurodevelopmental disorders. Archives of Neurology, 40: 407-411.
Weinberger DR: From neuropathology to neurodevelopment. Lancet 1995;346:552-57.
Weis S, Kimbacher M, Wegner E (1993) Morphometric analysis of the corpus callosum using MR: correlation of measurements with aging in healthy individuals. American Journal of Neuroradiology, 14: 637-645.
Weisbrod M, Winkler S, Maier S, Hill H, Thomas C, Spitzer M: Left lateralized P300 amplitude deficit in schizophrenic patients depends on pitch disparity. Biol Psychiatry 1997;41:541-9.
West MJ, Coleman PD, Flood DG, Troncoso JC (1994). Differences in the Pattern of Hippocampal Neuronal Loss in Normal Aging and Alzheimer's Disease, Lancet 344:769-772.
Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR (1981). Alzheimer's Disease: Evidence for Selective Loss of Cholinergic Neurons in the Nucleus Basalis, Ann. Neurol. 10:122-126.
Wilcock GK, Esiri MM (1982). Plaques, Tangles and Dementia: A Quantitative Study, J. Neurol. Sci. 56:343-356.
Witelson SF The brain connection: the corpus callosum is larger in left-handers. Science 1985 Aug 16;229(4714):665-668.
Witelson SF, Kigar DL (1992). Sylvian Fissure Morphology and Asymmetry in Men and Women: Bilateral Differences in Relation to Handedness in Men, J. Comp. Neurol. 323:326-340.
Witelson SF. Sex differences in neuroanatomical changes with aging. N Engl J Med. 1991 Jul 18; 325(3): 211-212.
Witelson SF: Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain 1989;112:799-835.
Woodruff PWR, McManus IC, David AS: Meta-analysis of corpus callosum size in schizophrenia. Journal of Neurology, Neurosurgery and Psychiatry 1995;58: 457-61.
Woodruff PWR, Pearlson GD, Geer MJ, Barta PE, Chilcoat HD: A computerized imaging study of corpus callosum morphology in schizophrenia. Psychological Medicine 1993;23:45-56.
Woodruff PWR, Phillips ML, Rushe T, Wright RC, Murray RM, David AS: Corpus callosum size and inter-hemispheric function in schizophrenia. Schizophrenia Res 1997;23:189-196.
Woods RP, Mazziotta JC, Cherry SR (1993). Automated Image Registration. In: Quantification of Brain Function. Tracer Kinetics and Image Analysis in Brain PET. K. Uemura et al., eds. Elsevier Science Publishers. 1993:391-398.
Worsley KJ (1994a). Quadratic Tests for Local Changes in Random Fields with Applications to Medical Images, Technical Report, Department of Mathematics and Statistics, McGill University, 94-08.
Worsley KJ (1994b). Local Maxima and the Expected Euler Characteristic of Excursion Sets of Chi-squared, F and t Fields, Advances in Applied Probability, 26:13-42.
Yakovlev PI, Lecours AP (1967) Regional Development of the Brain. Oxford: Blackwell.
Yamanouchi H, Sugiura S, Shimada H Loss of nerve fibres in the corpus callosum of progressive subcortical vascular encephalopathy J Neurol 1990 Feb;237(1):39-41.
Yoshii F, Shinohara Y, Duara R (1990). Cerebral White Matter Bundle Alterations in Patients with Dementia of Alzheimer Type and Patients with Multi-Infarct Dementia--Magnetic Resonance Imaging Study, Rinsho Shinkeigaku 30(1):110-112.
Paul Thompson
| RESUME| E-MAIL ME| PERSONAL HOMEPAGE| PROJECTS |
|---|