
Institution: UCLA Biomedical Library Serials
|| Sign In as Personal Subscriber
||
Contact Subscription Administrator at this Site
|| FAQ
Cerebral Cortex, Vol. 11, No. 1, 1-16,
January 2001
© 2001 Oxford University Press
Cortical Change in Alzheimer's Disease Detected with a Disease-specific Population-based Brain Atlas
Paul M. Thompson1,
Michael S. Mega1,2,
Roger P. Woods1,
Chris I. Zoumalan1,
Chris J. Lindshield1,
Rebecca E. Blanton1,
Jacob Moussai1,
Colin J. Holmes1,
Jeffrey L. Cummings2 and
Arthur W. Toga1
1 Laboratory of Neuro Imaging, Department of Neurology, Division of Brain Mapping and
2 Alzheimer's Disease Center, UCLA School of Medicine, Los Angeles, CA, USA
 |
Abstract
|
|---|
We report the first detailed population-based maps of cortical gray matter loss in Alzheimer's disease (AD), revealing prominent features of early structural change. New computational approaches were used to: (i) distinguish variations in gray matter distribution from variations in gyral patterns; (ii) encode these variations in a brain atlas (n = 46); (iii) create detailed maps localizing gray matter differences across groups. High resolution 3D magnetic resonance imaging (MRI) volumes were acquired from 26 subjects with mild to moderate AD (age 75.8 ± 1.7 years, MMSE score 20.0 ± 0.9) and 20 normal elderly controls (72.4 ± 1.3 years) matched for age, sex, handedness and educational level. Image data were aligned into a standardized coordinate space specifically developed for an elderly population. Eighty-four anatomical models per brain, based on parametric surface meshes, were created for all 46 subjects. Structures modeled included: cortical surfaces, all major superficial and deep cortical sulci, callosal and hippocampal surfaces, 14 ventricular regions and 36 gyral boundaries. An elastic warping approach, driven by anatomical features, was then used to measure gyral pattern variations. Measures of gray matter distribution were made in corresponding regions of cortex across all 46 subjects. Statistical variations in cortical patterning, asymmetry, gray matter distribution and average gray matter loss were then encoded locally across the cortex. Maps of group differences were generated. Average maps revealed complex profiles of gray matter loss in disease. Greatest deficits (20–30% loss, P < 0.001–0.0001) were mapped in the temporo-parietal cortices. The sensorimotor and occipital cortices were comparatively spared (0–5% loss, P > 0.05). Gray matter loss was greater in the left hemisphere, with different patterns in the heteromodal and idiotypic cortex. Gyral pattern variability also differed in cortical regions appearing at different embryonic phases. 3D mapping revealed profiles of structural deficits consistent with the cognitive, metabolic and histological changes in early AD. These deficits can therefore be (i) charted in a living population and (ii) compared across individuals and groups, facilitating longitudinal, genetic and interventional studies of dementia.
 |
Introduction
|
|---|
Considerable research has focused on uncovering specific patterns of cortical change in Alzheimer's disease (AD) and other dementias (Friedland and Luxenberg, 1988 ), schizophrenia (Kikinis et al., 1994
), schizophrenia (Kikinis et al., 1994 ; Csernansky et al., 1998
; Csernansky et al., 1998 ), epilepsy (Cook et al., 1994
), epilepsy (Cook et al., 1994 ), attention deficit hyperactivity disorder (ADHD) (Giedd et al., 1994
), attention deficit hyperactivity disorder (ADHD) (Giedd et al., 1994 ), autism (Filipek et al., 1989
), autism (Filipek et al., 1989 ; Courchesne, 1997
; Courchesne, 1997 ) and cortical dysplasias (Sobire et al., 1995
) and cortical dysplasias (Sobire et al., 1995 ) as well as normal development (Thompson et al., 2000b
) as well as normal development (Thompson et al., 2000b ). In AD early neuronal loss occurs in the entorhinal, parahippocampal and temporo-parietal cortex, consistent with the spatial pattern of early perfusion deficits and metabolic change. These deficits mirror the time course of cognitive impairment, proceeding from the entorhinal, temporal and perisylvian association cortices into more anterior regions as the disease progresses. In principle, volumetric magnetic resonance imaging (MRI) scans have sufficient resolution and tissue contrast to track cortical gray matter loss in a living individual. Yet, gyral patterns are extremely variable across subjects, making it difficult to calibrate individual patterns of gray matter loss against a normative population. It is also hard to determine the average profile of early tissue loss in a group. If 3D profiles of gray matter could be compared, this could be useful (i) for early diagnosis and assessing disease modification in an individual or group and (ii) for understanding how cortical changes relate to the fundamental anatomy of the cortex. This paper addresses these problems. It offers an approach to compare a patient's cortical anatomy and gray matter distribution with a population-based control image database. The approach is used to resolve group-specific patterns of gray matter distribution and cortical organization. The maps are made by first creating a brain atlas that encodes information on anatomical variability and cortical gray matter distribution in a population (Mazziotta et al., 1995
). In AD early neuronal loss occurs in the entorhinal, parahippocampal and temporo-parietal cortex, consistent with the spatial pattern of early perfusion deficits and metabolic change. These deficits mirror the time course of cognitive impairment, proceeding from the entorhinal, temporal and perisylvian association cortices into more anterior regions as the disease progresses. In principle, volumetric magnetic resonance imaging (MRI) scans have sufficient resolution and tissue contrast to track cortical gray matter loss in a living individual. Yet, gyral patterns are extremely variable across subjects, making it difficult to calibrate individual patterns of gray matter loss against a normative population. It is also hard to determine the average profile of early tissue loss in a group. If 3D profiles of gray matter could be compared, this could be useful (i) for early diagnosis and assessing disease modification in an individual or group and (ii) for understanding how cortical changes relate to the fundamental anatomy of the cortex. This paper addresses these problems. It offers an approach to compare a patient's cortical anatomy and gray matter distribution with a population-based control image database. The approach is used to resolve group-specific patterns of gray matter distribution and cortical organization. The maps are made by first creating a brain atlas that encodes information on anatomical variability and cortical gray matter distribution in a population (Mazziotta et al., 1995 ; Thompson et al., 1997
; Thompson et al., 1997 , 1998
, 1998 , 2000; Grenander and Miller, 1998
, 2000; Grenander and Miller, 1998 ).
).
We use a new computational strategy to compute gyral pattern variations across subjects. This approach is central to the study. It was used, rather than a more conventional stereotaxic approach, so that profiles of gray matter loss could be related to the gyral anatomy of each individual and not be confounded by the spatial variability within a stereotaxic template. Measures of gray matter made in each subject could then be averaged across regions of cortex that corresponded anatomically, rather than just stereotaxically. The resulting maps allow measures of gray matter loss to be plotted relative to the gyral anatomy of the cortex. Profiles of gray matter loss (and the spatial variability of the cortical pattern) can then be plotted on average models of the cortex for each group. In these average maps gyral features are well resolved and appear in their mean spatial locations. In this way, profiles of early gray matter loss are mathematically separated from underlying differences in cortical patterns and registration mismatch. Group differences are then displayed visually, using color coded 3D maps.
Hypotheses
We hypothesized that population-based averaging of anatomy would reveal a region of earliest tissue loss in the left temporal and parietal cortices, with a comparative sparing of the sensorimotor and occipital cortices. We also predicted that the temporoparietal cortices, specifically the left perisylvian language regions, would exhibit the greatest spatial variability, making it difficult to resolve these early structural changes without specialized approaches to control for such high anatomical variance (Thompson et al., 1997 ). These new approaches were also designed to allow mapping of cortical asymmetries important in evaluating evidence for the asymmetrical progression of the disease. In this way, true differences in gray matter distribution and cortical metabolism can be distinguished from individual or hemispheric differences in cortical organization.
). These new approaches were also designed to allow mapping of cortical asymmetries important in evaluating evidence for the asymmetrical progression of the disease. In this way, true differences in gray matter distribution and cortical metabolism can be distinguished from individual or hemispheric differences in cortical organization.
 |
Materials and Methods
|
|---|
Subjects and Image Acquisition
Imaging
High resolution 3D MRI volumes were acquired from 26 subjects diagnosed with mild to moderate AD and 20 elderly control subjects. Image volumes were acquired on a GE Signa 1.5 T clinical scanner (Milwaukee, WI). The scans were T1-weighted fast SPGR (spoiled GRASS) images with a 256 x 256 x 124 imaging matrix. Acquisition parameters were: TR/TE 14.3/3.2 ms, flip angle 35°, number of excitations 1, field of view 25 cm and contiguous 1.2 mm thick slices (no interslice gap) covering the entire brain. The resulting in-plane pixel resolution was 0.9765 x 0.9765 mm, sufficient for resolving the detailed anatomy of the cortex.
Diagnosis
The 26 patients met the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS-ARDRA) criteria for AD (McKhann et al., 1984 ). In addition, the patients had an acquired persistent decline involving at least three of the following domains: language, memory, visuospatial skills, cognition, emotion or personality (Cummings et al., 1980
). In addition, the patients had an acquired persistent decline involving at least three of the following domains: language, memory, visuospatial skills, cognition, emotion or personality (Cummings et al., 1980 ). Exclusion criteria for all subjects were the presence of a focal lesion on brain MRI, history of head trauma, past psychiatric history or an active medical problem.
). Exclusion criteria for all subjects were the presence of a focal lesion on brain MRI, history of head trauma, past psychiatric history or an active medical problem.
Demographics
Patients were matched for age (75.8 ± 1.7 years, 14 females/12 males), educational level (15.2 ± 0.4 years), disease severity and handedness (all right-handed). Their mean Mini-Mental State Exam score of 20.0 ± 0.9 (maximum score 30) (Folstein et al., 1975 ) was carefully matched across the patient cohort to reflect the mild AD population typically presenting initially to a clinic (Murphy et al., 1993
) was carefully matched across the patient cohort to reflect the mild AD population typically presenting initially to a clinic (Murphy et al., 1993 ). The 20 elderly control subjects were matched with the patients for age, sex, educational level and handedness (mean age 72.4 ± 1.3 years, 8 females/12 males, mean educational level 15.4 ± 0.5 years, all right-handed).
). The 20 elderly control subjects were matched with the patients for age, sex, educational level and handedness (mean age 72.4 ± 1.3 years, 8 females/12 males, mean educational level 15.4 ± 0.5 years, all right-handed).
Image Alignment and Pre-processing
3D Image Alignment
Imaging data were first aligned to a standard anatomical image template, specially constructed to reflect the average morphology of an elderly population. The construction of this template has been described in detail (Thompson et al., 2000a ). It forms the core of a growing disease-specific atlas of the brain in AD (Thompson et al., 2000a
). It forms the core of a growing disease-specific atlas of the brain in AD (Thompson et al., 2000a ,b
,b ,c
,c ; Mega et al., 1997, 1998
; Mega et al., 1997, 1998 , 2000a
, 2000a ,b
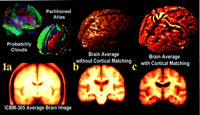
,b ). Briefly, the average MRI brain template (Fig. 1c
). Briefly, the average MRI brain template (Fig. 1c ) was constructed to have the average shape and size for a group of elderly subjects. Specialized approaches for anatomical averaging were used to generate an average MRI scan with well-resolved cortical features in their mean spatial locations. The resulting template reflects the average morphology of an elderly group of subjects and better reflects the anatomy of the subjects in this study than imaging templates based on young normals (Evans et al., 1994
) was constructed to have the average shape and size for a group of elderly subjects. Specialized approaches for anatomical averaging were used to generate an average MRI scan with well-resolved cortical features in their mean spatial locations. The resulting template reflects the average morphology of an elderly group of subjects and better reflects the anatomy of the subjects in this study than imaging templates based on young normals (Evans et al., 1994 ) (Fig. 1a
) (Fig. 1a ) or post-mortem data (Talairach and Tournoux, 1988
) or post-mortem data (Talairach and Tournoux, 1988 ).
).
View larger version (60K):
[in this window]
[in a new window]
|
Figure 1. Elderly brain template. In this study all 46 scans were aligned to an imaging template based on the anatomy of elderly subjects (c). This template is shown here for comparison with a widely used average brain image template (ICBM305) based on young normals (a). In the ICBM305 template, which was created by pixel-by-pixel intensity averaging of 305 young normal subjects' scans (Evans et al., 1994 ), notice how anatomical features are not well resolved at the cortex. Cortical variability is represented using probability clouds (top left) that describe the frequency of incidence for each gyrus at each stereotaxic voxel, after linear registration and voxel-by-voxel comparison. In a brain template (b) similarly constructed from nine AD patients' scans the cortical average is also poorly resolved. In contrast, anatomical features are highly resolved, even at the cortex, in the Continuum-Mechanical Brain Template (c), which applies a cortical matching transformation to each brain before intensity averaging (Thompson et al., 2000a ), notice how anatomical features are not well resolved at the cortex. Cortical variability is represented using probability clouds (top left) that describe the frequency of incidence for each gyrus at each stereotaxic voxel, after linear registration and voxel-by-voxel comparison. In a brain template (b) similarly constructed from nine AD patients' scans the cortical average is also poorly resolved. In contrast, anatomical features are highly resolved, even at the cortex, in the Continuum-Mechanical Brain Template (c), which applies a cortical matching transformation to each brain before intensity averaging (Thompson et al., 2000a ). Scans are elastically reconfigured into a group mean configuration, using surface-based warping to match 84 surface models (including gyral pattern elements) across all subjects. The intensities of the reconfigured scans are then averaged voxel-by-voxel, after intensity normalization. This produces a group image template with the average geometry and average image intensity for the group. Elastic transformations are required to resolve cortical features, in their mean configuration, after scans are averaged together (cf. Grenander and Miller, 1998). The data in this study were aligned to this specially constructed elderly image template, which better reflects elderly anatomy than templates based on young normals. ). Scans are elastically reconfigured into a group mean configuration, using surface-based warping to match 84 surface models (including gyral pattern elements) across all subjects. The intensities of the reconfigured scans are then averaged voxel-by-voxel, after intensity normalization. This produces a group image template with the average geometry and average image intensity for the group. Elastic transformations are required to resolve cortical features, in their mean configuration, after scans are averaged together (cf. Grenander and Miller, 1998). The data in this study were aligned to this specially constructed elderly image template, which better reflects elderly anatomy than templates based on young normals.
|
|
Imaging data were aligned to this template using Automated Image Registration software (Woods et al., 1993 , 1998
, 1998 ). An affine (linear) transformation was applied to individual datasets to transform them into our common coordinate space. This standard alignment was necessary for automated extraction of anatomical structure models and generation of tissue maps.
). An affine (linear) transformation was applied to individual datasets to transform them into our common coordinate space. This standard alignment was necessary for automated extraction of anatomical structure models and generation of tissue maps.
RF Inhomogeneity Correction
MRI volumes were corrected for potential non-uniformities in MR signal intensity due to field inhomogeneities in the scanner. An automated algorithm (Zijdenbos and Dawant, 1994 ; Sled et al., 1998
; Sled et al., 1998 ) derived a 3D map of these low frequency signal fluctuations and divided by this scalar field to correct for any errors at the voxel level.
) derived a 3D map of these low frequency signal fluctuations and divided by this scalar field to correct for any errors at the voxel level.
Tissue Classification and Mapping
For all 46 subjects maps of gray matter, white matter and cerebrospinal fluid (CSF) were generated, so that regional differences in gray matter could be identified. Briefly, samples of each tissue class were interactively tagged to compute the parameters of a Gaussian mixture distribution that reflects statistical variability in the intensity of each tissue type (Sled et al., 1998 ). A nearest neighbor tissue classifier then assigned each image voxel to a particular tissue class (gray, white or CSF) or to a background class (representing extracerebral voxels in the image). The inter/intra-rater reliability of this protocol and its robustness to changes in image acquisition parameters have been described previously (Sowell et al., 1999a
). A nearest neighbor tissue classifier then assigned each image voxel to a particular tissue class (gray, white or CSF) or to a background class (representing extracerebral voxels in the image). The inter/intra-rater reliability of this protocol and its robustness to changes in image acquisition parameters have been described previously (Sowell et al., 1999a ,b
,b ). Gray matter maps were retained for subsequent analysis.
). Gray matter maps were retained for subsequent analysis.
Cortical Surface Extraction
Next, a high resolution shape representation of the cortex was automatically extracted for each subject, as described previously (Thompson et al., 1997 ). This algorithm successively deforms a spherical surface into the configuration of a given subject's cortex, resolving the gyral pattern. The ability of the 3D active surface extraction algorithm (MacDonald et al., 1993
). This algorithm successively deforms a spherical surface into the configuration of a given subject's cortex, resolving the gyral pattern. The ability of the 3D active surface extraction algorithm (MacDonald et al., 1993 , 1994
, 1994 , 1998) to extract high fidelity surface representations of the CSF/gray matter and gray/white matter interfaces in high resolution MR data has been extensively tested and validated in prior studies (Holmes et al., 1996
, 1998) to extract high fidelity surface representations of the CSF/gray matter and gray/white matter interfaces in high resolution MR data has been extensively tested and validated in prior studies (Holmes et al., 1996 ; Thompson et al., 1997
; Thompson et al., 1997 , 1998
, 1998 ; MacDonald, 1998
; MacDonald, 1998 ). The resulting cortical model consists of a mesh of discrete triangular elements that tile the surface. The intensity value at which the gray matter/CSF interface occurred was determined in 20–30 cortical regions and the threshold set to the mean. This threshold was independently validated in each case by comparing the automatically extracted surface boundaries with the manually determined surface of the gray matter–CSF boundary, identified at high magnification in the corresponding 3D MR volumes.
). The resulting cortical model consists of a mesh of discrete triangular elements that tile the surface. The intensity value at which the gray matter/CSF interface occurred was determined in 20–30 cortical regions and the threshold set to the mean. This threshold was independently validated in each case by comparing the automatically extracted surface boundaries with the manually determined surface of the gray matter–CSF boundary, identified at high magnification in the corresponding 3D MR volumes.
Gyral Pattern Modeling
To determine the patterns of variability for individual regions of cortex, 36 additional cortical structures per brain were traced in all 46 subjects. These 36 major external fissures and sulci in the brain (Table 1 ) were manually outlined on a highly magnified surface-rendered image of each cortex. Priority was given to biological features whose topological consistency has been demonstrated across normal populations (Ono et al., 1990
) were manually outlined on a highly magnified surface-rendered image of each cortex. Priority was given to biological features whose topological consistency has been demonstrated across normal populations (Ono et al., 1990 ; Le Goualher et al., 1996
; Le Goualher et al., 1996 ; MacDonald et al., 1997
; MacDonald et al., 1997 ). Detailed anatomical criteria were applied as previously set out (Steinmetz et al., 1989
). Detailed anatomical criteria were applied as previously set out (Steinmetz et al., 1989 , 1990
, 1990 ; Missir et al., 1989
; Missir et al., 1989 ; Leonard, 1996
; Leonard, 1996 ; Thompson and Toga, 1997
; Thompson and Toga, 1997 ; Thompson et al., 1997
; Thompson et al., 1997 ; Kennedy et al., 1998
; Kennedy et al., 1998 ) and as in a sulcal atlas (Ono et al., 1990
) and as in a sulcal atlas (Ono et al., 1990 ). In both hemispheres 3D curves were drawn to represent the superior and inferior frontal, central, post-central, intraparietal, superior and inferior temporal, collateral, olfactory and occipito-temporal sulci, as well as the Sylvian fissures. Additional 3D curves were drawn to represent gyral limits at the interhemispheric margin1 (Thompson et al., 1997
). In both hemispheres 3D curves were drawn to represent the superior and inferior frontal, central, post-central, intraparietal, superior and inferior temporal, collateral, olfactory and occipito-temporal sulci, as well as the Sylvian fissures. Additional 3D curves were drawn to represent gyral limits at the interhemispheric margin1 (Thompson et al., 1997 ). Stereotaxic locations of contour points derived from the data volume were re-digitized to produce 36 uniformly parameterized cortical contours per brain, representing the primary gyral pattern of each subject (Thompson et al., 1997
). Stereotaxic locations of contour points derived from the data volume were re-digitized to produce 36 uniformly parameterized cortical contours per brain, representing the primary gyral pattern of each subject (Thompson et al., 1997 ; Thompson and Toga, 1998
; Thompson and Toga, 1998 ).
).
3D Cortical Surface Averaging
Transforming individual data into a standardized coordinate space removes differences in overall brain size. Nonetheless, substantial anatomical variability remains, especially at the cortex, due to individual differences in gyral patterning (Steinmetz et al., 1989 , 1990
, 1990 ; Thompson et al., 1996b
; Thompson et al., 1996b ) and disease-related atrophy (Mega et al., 1998
) and disease-related atrophy (Mega et al., 1998 ). Information was stored on individual cortical differences by (i) creating average geometric models for the cortex and then (ii) measuring individual deviations from the group average using 3D displacement maps (Fig. 2
). Information was stored on individual cortical differences by (i) creating average geometric models for the cortex and then (ii) measuring individual deviations from the group average using 3D displacement maps (Fig. 2 ) (Thompson et al., 1996a
) (Thompson et al., 1996a ,b
,b , 1997
, 1997 ; Thompson and Toga, 1997
; Thompson and Toga, 1997 ; Davatzikos et al., 1996
; Davatzikos et al., 1996 ; Mega et al., 1998
; Mega et al., 1998 ; Csernansky et al., 1998
; Csernansky et al., 1998 ). These displacement maps relate points on an individual subject's cortex to corresponding points on an average cortical model. Average cortical model construction has been described previously (Ge et al., 1995
). These displacement maps relate points on an individual subject's cortex to corresponding points on an average cortical model. Average cortical model construction has been described previously (Ge et al., 1995 ; Collins et al., 1996
; Collins et al., 1996 ; Drury and Van Essen, 1997
; Drury and Van Essen, 1997 ; Thompson et al., 1997
; Thompson et al., 1997 , 2000a
, 2000a ; Fischl et al., 1999
; Fischl et al., 1999 ) (see Fig. 2
) (see Fig. 2 and Appendix for a summary).
and Appendix for a summary).

View larger version (66K):
[in this window]
[in a new window]
|
Figure 2. Computing differences in cortical patterns. Cortical anatomy can be compared, for any pair of subjects (3D models; top left), by computing the 3D deformation field that reconfigures one subject's cortex onto another (right panel). In this mapping gyral patterns must also be constrained to match their counterparts in the target brain. To do this, the algorithm that automatically extracts a 3D model of the cortex provides a continuous inverse mapping from each subject's cortex to a sphere or plane. A flow field in the flat parameter space then drives the cortical features into register (c,d). The full mapping (bottom right) can be recovered in 3D space as a displacement vector field that drives cortical points and regions in one brain into precise structural registration with their counterparts in the other brain. Gyral patterns can also be matched across a group of subjects to create average cortical surfaces. (c) A cortical flat map for a hemisphere of one subject, with the average cortical pattern for the group overlaid (colored lines). (d) The result of warping the individual's sulcal pattern into the average configuration for the group, using the covariant flow equations (Thompson et al., 2000a ). The 3D cortical regions that map to these average locations are then recovered in each individual subject, as follows. A color code (Thompson and Toga, 1997 ). The 3D cortical regions that map to these average locations are then recovered in each individual subject, as follows. A color code (Thompson and Toga, 1997 ) representing 3D cortical point locations (e) in this subject is convected along with the flow that drives the sulcal pattern into the average configuration for the group (d). Once this is done for all subjects, points on each individual's cortex are recovered (f,g) that have the same relative location to the primary folding pattern in all subjects. Averaging of these corresponding points results in a crisp average cortex. The transformation fields that map individuals onto the group average (h) are stored and used to measure regional variability. ) representing 3D cortical point locations (e) in this subject is convected along with the flow that drives the sulcal pattern into the average configuration for the group (d). Once this is done for all subjects, points on each individual's cortex are recovered (f,g) that have the same relative location to the primary folding pattern in all subjects. Averaging of these corresponding points results in a crisp average cortex. The transformation fields that map individuals onto the group average (h) are stored and used to measure regional variability.
|
|
To allow point-to-point cortical averaging, each subject's cortical model is converted to a ‘flat map’ as previously described (Thompson et al., 2000a ). To ensure that each subject's flat map can also be converted back into a 3D cortical model, cortical surface point position vectors in 3D stereotaxic space were represented on the flat map using a color code as described previously (Thompson et al., 2000a
). To ensure that each subject's flat map can also be converted back into a 3D cortical model, cortical surface point position vectors in 3D stereotaxic space were represented on the flat map using a color code as described previously (Thompson et al., 2000a ). This forms an image of the parameter space in RGB color image format (Fig. 2e
). This forms an image of the parameter space in RGB color image format (Fig. 2e ). By carrying a color code (that indexes 3D locations) along with the vector flow that aligns each individual with the average folding pattern (Fig. 2c,d
). By carrying a color code (that indexes 3D locations) along with the vector flow that aligns each individual with the average folding pattern (Fig. 2c,d ), information can be recovered at a particular location in the average folding pattern (Fig. 2f
), information can be recovered at a particular location in the average folding pattern (Fig. 2f ) specifying the 3D cortical points mapping each subject to the average. The resulting mapping is guaranteed to average together all points falling on the same cortical locations across the set of brains and ensures that corresponding features are averaged together (Fig. 3
) specifying the 3D cortical points mapping each subject to the average. The resulting mapping is guaranteed to average together all points falling on the same cortical locations across the set of brains and ensures that corresponding features are averaged together (Fig. 3 ). It can also be determined which regions of the cortex show the greatest variability in structure. By using the color code (Fig. 2f
). It can also be determined which regions of the cortex show the greatest variability in structure. By using the color code (Fig. 2f ) to identify original cortical locations in 3D space (Fig. 2g
) to identify original cortical locations in 3D space (Fig. 2g ), displacement fields were recovered mapping each subject into gyrus-by-gyrus correspondence with the average cortex (Fig. 4
), displacement fields were recovered mapping each subject into gyrus-by-gyrus correspondence with the average cortex (Fig. 4 ). Anatomical variability was defined at each point on the average cortical surface as the root mean square (r.m.s.) magnitude of the 3D displacement vectors assigned to each point in the surface maps from individual to average (Thompson et al., 1996a
). Anatomical variability was defined at each point on the average cortical surface as the root mean square (r.m.s.) magnitude of the 3D displacement vectors assigned to each point in the surface maps from individual to average (Thompson et al., 1996a ,b
,b , 1997
, 1997 ). This measure captures how individuals deviate from the group average anatomy after taking gyral pattern variations into account. The resulting variability pattern was visualized as a color coded map (Fig. 5
). This measure captures how individuals deviate from the group average anatomy after taking gyral pattern variations into account. The resulting variability pattern was visualized as a color coded map (Fig. 5 ).
).
View larger version (58K):
[in this window]
[in a new window]
|
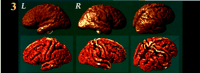
Figure 3. Average cortex in AD. The average cortical surface for the group is shown (bottom row) as a graphically rendered surface model. If sulcal position vectors are averaged without aligning the intervening gyral patterns (top), sulcal features are not reinforced across subjects and a smooth average cortex is produced. By matching gyral patterns across subjects before averaging, a crisper average cortex is produced (bottom row). Sulcal features that consistently occur across all subjects appear in their average geometric configuration.
|
|
View larger version (57K):
[in this window]
[in a new window]
|
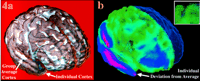
Figure 4. Matching an individual's cortex to the average cortex. 3D variability patterns across the cortex are measured by driving individual cortical patterns into local correspondence with the average cortical model. (a) How the anatomy of one subject (brown surface mesh) deviates from an average cortical model (white) after affine alignment of the individual data. (b) The deformation vector field required to reconfigure the gyral pattern of the subject into the exact configuration of the average cortex. The transformation is shown as a flow field that takes the individual's anatomy onto the right hemisphere of the average cortex (shown as a blue surface mesh). The largest amount of deformation is required in the temporal and parietal cortex (pink, large deformation). Details of the 3D vector deformation field (b, inset) show the local complexity of the mapping. Storage of these mappings allows quantification of local anatomical variability.
|
|
View larger version (54K):
[in this window]
[in a new window]
|
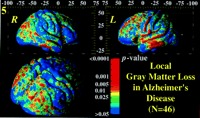
Figure 5. Statistical map of average gray matter loss in AD (n = 46). Based on averaging and comparing gray matter measurements across equivalent regions of cortex in all 46 subjects, this statistical field reflects whether the average gray matter is reduced in patients (average of 26 subjects) relative to controls (average of 20 subjects). The significance of this reduction at each cortical location is shown. Severe, more localized reductions are visualized in the temporal lobe and temporo-parietal cortex. This profile of gray matter loss mirrors the anatomical distribution of early perfusion deficits and metabolic change in mild to moderate AD.
|
|
Gray Matter Averaging and Statistical Comparisons of Gray Matter Distribution
Gray Matter Quantification
Given that the deformation maps associate cortical locations with the same relation to the primary folding pattern across subjects, a local measurement of gray matter density was made in each subject and averaged across equivalent cortical locations. To quantify local gray matter, we used a measure termed ‘gray matter density’, which has been used in prior studies to compare the spatial distribution of gray matter across subjects (Sowell et al., 1999a ,b
,b ; Thompson et al., 2000d
; Thompson et al., 2000d ). This measures the proportion of gray matter in a small region of fixed radius around a point. In these prior studies, however, it was assumed that gray matter occurring at the same stereotaxic location came from equivalent anatomical regions across subjects. Given the large anatomical variability in some cortical regions (Fig. 5
). This measures the proportion of gray matter in a small region of fixed radius around a point. In these prior studies, however, it was assumed that gray matter occurring at the same stereotaxic location came from equivalent anatomical regions across subjects. Given the large anatomical variability in some cortical regions (Fig. 5 ), especially in a diseased population, this assumption is often violated. To avoid this potential methodological error we employed elastic maps to associate equivalent gyri across both populations. We were thus able to average gray matter density across corresponding cortical regions and plot the results on the continuum-mechanical average AD cortex (Fig. 3
), especially in a diseased population, this assumption is often violated. To avoid this potential methodological error we employed elastic maps to associate equivalent gyri across both populations. We were thus able to average gray matter density across corresponding cortical regions and plot the results on the continuum-mechanical average AD cortex (Fig. 3 ). Briefly, at each cortical point a sphere of radius 5 mm was made, centered at that point. By reference to the gray matter maps derived from the tissue classification approach described above, the proportion of gray matter pixels relative to the total number of pixels in this sphere was computed and stored as a map of gray matter densities across the cortex. Because this measure is just another cortical attribute that can be aligned across subjects, and with the mean gyral pattern for the group (Figs 3,4
). Briefly, at each cortical point a sphere of radius 5 mm was made, centered at that point. By reference to the gray matter maps derived from the tissue classification approach described above, the proportion of gray matter pixels relative to the total number of pixels in this sphere was computed and stored as a map of gray matter densities across the cortex. Because this measure is just another cortical attribute that can be aligned across subjects, and with the mean gyral pattern for the group (Figs 3,4
 ), the average gray matter density was computed across subjects for each cortical point in each group average. Maps of average gray matter loss were also created by comparing the average maps from the diseased and control groups. Finally, information was stored on the variability in gray matter density at equivalent cortical locations within and across groups. This statistical data within each group allowed the observed profiles of average gray matter difference to be calibrated against a variance measure for the index. This variance measure allowed the significance of local gray matter reductions to be assessed. A field of test statistics was attached to the average surface for the diseased group to determine the local statistical significance of the hypothesized gray matter loss. Finally, a localized test for gray matter loss in the temporo-parietal cortex was applied, a region where greatest neuronal loss was hypothesized at this early stage of the disease.
), the average gray matter density was computed across subjects for each cortical point in each group average. Maps of average gray matter loss were also created by comparing the average maps from the diseased and control groups. Finally, information was stored on the variability in gray matter density at equivalent cortical locations within and across groups. This statistical data within each group allowed the observed profiles of average gray matter difference to be calibrated against a variance measure for the index. This variance measure allowed the significance of local gray matter reductions to be assessed. A field of test statistics was attached to the average surface for the diseased group to determine the local statistical significance of the hypothesized gray matter loss. Finally, a localized test for gray matter loss in the temporo-parietal cortex was applied, a region where greatest neuronal loss was hypothesized at this early stage of the disease.
Computer Platform
All algorithms were written in C and executed on Silicon Graphics O2 R10000 workstations running IRIX 6.5, except for the algorithms for cortical extraction and matching, which were parallelized and executed on a networked cluster of 14 workstations and a Silicon Graphics RealityMonster with 32 internal processors.
 |
Results
|
|---|
Cortical Gray Matter Distribution and Disease–Related Gray Matter Loss
Figure 5 shows a surface-based probability field that indicates the regional significance of gray matter loss across the cortex in the entire AD cohort. Red (P < 0.005) denotes brain regions where the average gray matter index is significantly less2 in the AD cohort than in the control group. All averages and comparisons are made across corresponding areas of cortex, defined by gyral pattern matching (Fig. 4
shows a surface-based probability field that indicates the regional significance of gray matter loss across the cortex in the entire AD cohort. Red (P < 0.005) denotes brain regions where the average gray matter index is significantly less2 in the AD cohort than in the control group. All averages and comparisons are made across corresponding areas of cortex, defined by gyral pattern matching (Fig. 4 ). Given these statistics, two types of inference are possible. First, the a priori hypothesis of gray matter loss in the temporal and parietal cortex was confirmed. There was also evidence for a region of maximal loss throughout the lateral temporal surface and the parietal operculum bilaterally (P < 0.001–0.00012).
). Given these statistics, two types of inference are possible. First, the a priori hypothesis of gray matter loss in the temporal and parietal cortex was confirmed. There was also evidence for a region of maximal loss throughout the lateral temporal surface and the parietal operculum bilaterally (P < 0.001–0.00012).
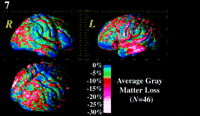
A pervasive left greater than right hemisphere reduction in gray matter was found (with up to 20–30% loss locally; see Fig. 7 ), consistent with the suggestion from metabolic studies (Loewenstein et al., 1989
), consistent with the suggestion from metabolic studies (Loewenstein et al., 1989 ) that the left hemisphere is, on average, more severely affected at this stage of the disease. The occipital cortices were comparatively spared bilaterally, as were the sensorimotor cortices (0–5% loss, P > 0.05). There was also severe gray matter loss (20–30%, P < 0.001–0.0001) in the middle frontal gyrus, in the vicinity of areas 9 and 46 (Rajkowska and Goldman-Rakic, 1995
) that the left hemisphere is, on average, more severely affected at this stage of the disease. The occipital cortices were comparatively spared bilaterally, as were the sensorimotor cortices (0–5% loss, P > 0.05). There was also severe gray matter loss (20–30%, P < 0.001–0.0001) in the middle frontal gyrus, in the vicinity of areas 9 and 46 (Rajkowska and Goldman-Rakic, 1995 ). We further investigated whether the regions of more significant gray matter loss reflected a correspondingly greater average reduction in the local gray matter index (Fig. 7
). We further investigated whether the regions of more significant gray matter loss reflected a correspondingly greater average reduction in the local gray matter index (Fig. 7 ). This was important, as a greater significance value can result either from (i) a genuinely greater percent reduction in the mean gray matter in AD or (ii) a local reduction in the variance of the gray matter index across the group, which translates into a greater detection sensitivity. Interestingly, a map of the percentage reduction in average gray matter (Fig. 7
). This was important, as a greater significance value can result either from (i) a genuinely greater percent reduction in the mean gray matter in AD or (ii) a local reduction in the variance of the gray matter index across the group, which translates into a greater detection sensitivity. Interestingly, a map of the percentage reduction in average gray matter (Fig. 7 ) followed approximately the same anatomical pattern, suggesting that there is indeed a hierarchy in the severity of gray matter loss at this stage of the disease, rather than a fluctuation in the local power of the statistical model to detect it. Again, the temporal and temporo-parietal cortex exhibited severe (10–30%) reductions in gray matter. This contrasted with a comparative sparing of the superior margins of the central and post-central gyri and occipital poles (0–5% loss). Although diffuse gray matter loss is likely to occur across the majority of the cortex, it is interesting that the superior central and post-central gyri and occipital poles show very little reduction in gray matter when adjacent posterior temporal cortex and the parietal operculum are severely affected, in both the percentage loss and statistical anatomical maps.
) followed approximately the same anatomical pattern, suggesting that there is indeed a hierarchy in the severity of gray matter loss at this stage of the disease, rather than a fluctuation in the local power of the statistical model to detect it. Again, the temporal and temporo-parietal cortex exhibited severe (10–30%) reductions in gray matter. This contrasted with a comparative sparing of the superior margins of the central and post-central gyri and occipital poles (0–5% loss). Although diffuse gray matter loss is likely to occur across the majority of the cortex, it is interesting that the superior central and post-central gyri and occipital poles show very little reduction in gray matter when adjacent posterior temporal cortex and the parietal operculum are severely affected, in both the percentage loss and statistical anatomical maps.
View larger version (44K):
[in this window]
[in a new window]
|
Figure 7. Map of average gray matter loss in AD expressed as a percentage of average control values (n = 46). This map expresses the same data as Figure 5 as a percentage reduction in the measurement of gray matter when equivalent cortical regions are averaged and compared between AD patients and controls. As hypothesized, pervasive left greater than right reductions are mapped. The percentage reduction in average gray matter followed approximately the same anatomical pattern as the significance map, suggesting that there is indeed a hierarchy in the severity of gray matter loss at this stage of the disease, rather than a fluctuation in the local power of the statistical model to detect it. Again, the temporal and temporo-parietal cortex exhibited severe (10–30%) reductions in gray matter. This contrasted with a comparative sparing of the superior margins of the central and post-central gyri and the occipital poles (0–5% loss). as a percentage reduction in the measurement of gray matter when equivalent cortical regions are averaged and compared between AD patients and controls. As hypothesized, pervasive left greater than right reductions are mapped. The percentage reduction in average gray matter followed approximately the same anatomical pattern as the significance map, suggesting that there is indeed a hierarchy in the severity of gray matter loss at this stage of the disease, rather than a fluctuation in the local power of the statistical model to detect it. Again, the temporal and temporo-parietal cortex exhibited severe (10–30%) reductions in gray matter. This contrasted with a comparative sparing of the superior margins of the central and post-central gyri and the occipital poles (0–5% loss).
|
|
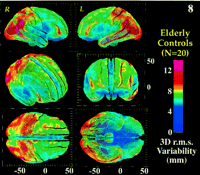
Cortical Pattern Variability
As a by-product of the gray matter analysis, maps revealing the magnitude and directional biases of 3D normal cortical variability are shown in Figures 8 and 9
 . For each cortical region principal directions emerged in which the magnitude of normal cortical variability was greatest (Fig. 9
. For each cortical region principal directions emerged in which the magnitude of normal cortical variability was greatest (Fig. 9 ). The overall magnitude of variability was also highly heterogeneous. In the control subjects (n = 20; Fig. 8
). The overall magnitude of variability was also highly heterogeneous. In the control subjects (n = 20; Fig. 8 ) variability values rose from 4–5 mm in the primary motor cortex to localized peaks of maximum variability in the posterior perisylvian zones and superior frontal association cortex (12–14 mm). The primary sensory and motor areas showed a localized invariance relative to all other regions of the cortex, with bilateral r.m.s. variability values of 2–5 mm at the central sulcus rising only to 6–9 mm at the post-central sulcus in both brain hemispheres.
) variability values rose from 4–5 mm in the primary motor cortex to localized peaks of maximum variability in the posterior perisylvian zones and superior frontal association cortex (12–14 mm). The primary sensory and motor areas showed a localized invariance relative to all other regions of the cortex, with bilateral r.m.s. variability values of 2–5 mm at the central sulcus rising only to 6–9 mm at the post-central sulcus in both brain hemispheres.
View larger version (83K):
[in this window]
[in a new window]
|
Figure 8. 3D cortical variability (n = 20, normal elderly subjects). The profile of variability across the cortex is shown after differences in brain orientation and size were removed. The following views are shown: oblique frontal, frontal, right, left, top, bottom. Again, extreme variability in the posterior perisylvian zones and superior frontal association cortex (12–14 mm, red) contrasts with the comparative invariance of the primary sensory, motor and orbitofrontal cortex (2–5 mm, blue). The region of maximal variability, in the temporal cortex, is tightly linked with the location of human visual area MT (or V5) (Watson et al., 1993 ). Extreme caution is therefore necessary when referring to activation foci here using stereotaxic coordinates. The overall profiles of variation also corroborate recent volumetric findings based on a fine scale parcellation of the cortex (Kennedy et al., 1998 ). Extreme caution is therefore necessary when referring to activation foci here using stereotaxic coordinates. The overall profiles of variation also corroborate recent volumetric findings based on a fine scale parcellation of the cortex (Kennedy et al., 1998 ), with greater morphological individuality in phylogenetically more recent cortical regions. ), with greater morphological individuality in phylogenetically more recent cortical regions.
|
|
View larger version (55K):
[in this window]
[in a new window]
|
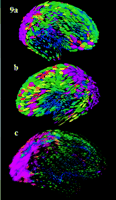
Figure 9. Tensor maps reveal directional biases in normal cortical variability (n = 20). Tensor maps can be used to visualize these complex patterns of gyral pattern variation at the cortex. The maps are based on the group of 20 elderly normal subjects. Color distinguishes regions of high variability (pink) from areas of low variability (blue). In (a) and (b) ellipsoidal glyphs indicate the principal directions of variation: they are most elongated along directions where there is greatest anatomical variation across subjects. Each glyph represents the covariance tensor of the vector fields that map individual subjects onto their group average anatomical representation. The resulting information can be leveraged to distinguish normal from abnormal anatomical variants using random field algorithms and can define statistical distributions for feature labeling at the cortex (Le Goualher et al., 1999; Vaillant and Davatzikos, 1999). (c) Probabilistic confidence limits on normal anatomical variation: tensor field representation. Again, tensor maps reveal the preferred directions of cortical variation, after sulcal pattern correspondences are taken into account. Variability is greatest in the temporo-parietal cortex. Since cortical variations are modeled as vector field displacements of an average cortical model, ellipsoids of constant probability density can be computed across cortical regions (relative to an average cortex). These probability fields are obtained by singular value decomposition, or Cholesky factorization, of the local covariance tensor (Thompson et al., 1996a ; Cao and Worsley, 2000 ; Cao and Worsley, 2000 ). Confidence ellipsoids are shown, colored by the determinant of the covariance tensor, which measures the magnitude of anatomical variability at each location. ). Confidence ellipsoids are shown, colored by the determinant of the covariance tensor, which measures the magnitude of anatomical variability at each location.
|
|
System-specific Variability Patterns
Extremely low variability values in the motor cortex (2–5 mm) rose with the transition anteriorly from motor area 4 to pre-frontal association cortex (see Fig. 8a ). Peak variability values (12–14 mm) occurred in the anterior frontal association cortex on the left and throughout the middle frontal gyrus on the right, where Brodmann area 46 is consistently located (Brodmann, 1909
). Peak variability values (12–14 mm) occurred in the anterior frontal association cortex on the left and throughout the middle frontal gyrus on the right, where Brodmann area 46 is consistently located (Brodmann, 1909 ; Rajkowska and Goldman-Rakic, 1995
; Rajkowska and Goldman-Rakic, 1995 ). In these regions of frontal cortex hemispheric differences in gyral organization are typical (Malobabic et al., 1993
). In these regions of frontal cortex hemispheric differences in gyral organization are typical (Malobabic et al., 1993 ). Moving inferiorly, intermediate variability values (6–10 mm) over the inferior prefrontal convexity fell with the transition to the orbitofrontal cortex, where the gyral pattern is highly conserved across subjects (2–5 mm variability). More laterally, the posterior frontal cortex, including territory occupied by Broca's area, also displayed intermediate variability (6–10 mm). Temporal lobe variability rose from 2–3 mm in the depths of the Sylvian cisternae to 18 mm at the posterior limit of the inferior temporal sulcus in both brain hemispheres (Fig. 8a
). Moving inferiorly, intermediate variability values (6–10 mm) over the inferior prefrontal convexity fell with the transition to the orbitofrontal cortex, where the gyral pattern is highly conserved across subjects (2–5 mm variability). More laterally, the posterior frontal cortex, including territory occupied by Broca's area, also displayed intermediate variability (6–10 mm). Temporal lobe variability rose from 2–3 mm in the depths of the Sylvian cisternae to 18 mm at the posterior limit of the inferior temporal sulcus in both brain hemispheres (Fig. 8a ). This suggests that the region of maximal variability in the human temporal cortex may lie posterior to the region of highest variability observed by Novikov and Podcherednik in primary auditory cortex (Novikov and Podcherednik, 1992
). This suggests that the region of maximal variability in the human temporal cortex may lie posterior to the region of highest variability observed by Novikov and Podcherednik in primary auditory cortex (Novikov and Podcherednik, 1992 ). Furthermore, in the vicinity of the angular gyrus the 3D r.m.s. variability of the inferior temporal sulci was substantially greater on the left (12–14 mm) than the right (10–12 mm). This left greater than right variability pattern was also displayed by the superior temporal sulcus, the supramarginal gyrus and the posterior ascending ramus of the Sylvian fissure, which was also considerably more variable on the left (12–14 mm), where Wernicke's area is situated, than on the right (6–10 mm). These findings of asymmetrical variability support earlier hypotheses by Steinmetz and co-workers, who examined 2-dimensional sagittal projections of the Sylvian fissure (Steinmetz et al., 1990
). Furthermore, in the vicinity of the angular gyrus the 3D r.m.s. variability of the inferior temporal sulci was substantially greater on the left (12–14 mm) than the right (10–12 mm). This left greater than right variability pattern was also displayed by the superior temporal sulcus, the supramarginal gyrus and the posterior ascending ramus of the Sylvian fissure, which was also considerably more variable on the left (12–14 mm), where Wernicke's area is situated, than on the right (6–10 mm). These findings of asymmetrical variability support earlier hypotheses by Steinmetz and co-workers, who examined 2-dimensional sagittal projections of the Sylvian fissure (Steinmetz et al., 1990 ).
).
Tensor Maps Reveal Directional Biases in Cortical Variability
For each region of cortex clear directional biases emerged in the principal directions of gyral pattern variability (Fig. 9a,b ). Gyral patterns did not vary equally in all directions and the statistical distribution that describes the location of a cortical region in space was elongated in a particular direction, which also varied locally across the cortex. To visualize this, cortical variations were modeled as vector field displacements of an average cortical model and ellipsoids of constant probability density were computed for positions of cortical regions (relative to the average cortex). Figure 9c
). Gyral patterns did not vary equally in all directions and the statistical distribution that describes the location of a cortical region in space was elongated in a particular direction, which also varied locally across the cortex. To visualize this, cortical variations were modeled as vector field displacements of an average cortical model and ellipsoids of constant probability density were computed for positions of cortical regions (relative to the average cortex). Figure 9c shows the shape of a 3D Gaussian distribution fitted at each point on the average normal cortex, reflecting the cross-subject variation of points from equivalent gyral regions. The shape of this distribution at each cortical point is described by the covariance tensor of the 3D distribution. Its value determines a set of nested ellipsoids that represent confidence limits for the locations of corresponding anatomical points in stereotaxic space (Thompson et al., 1997
shows the shape of a 3D Gaussian distribution fitted at each point on the average normal cortex, reflecting the cross-subject variation of points from equivalent gyral regions. The shape of this distribution at each cortical point is described by the covariance tensor of the 3D distribution. Its value determines a set of nested ellipsoids that represent confidence limits for the locations of corresponding anatomical points in stereotaxic space (Thompson et al., 1997 ; Cao and Worsley, 2000
; Cao and Worsley, 2000 ). These ellipsoids (Fig. 9c
). These ellipsoids (Fig. 9c ) are colored by the determinant of the covariance tensor, for which larger values (pink) represent greater 3D variability and small values (blue) represent regions whose morphology is highly conserved across subjects.
) are colored by the determinant of the covariance tensor, for which larger values (pink) represent greater 3D variability and small values (blue) represent regions whose morphology is highly conserved across subjects.
Anatomical variations in the temporo-parietal regions displayed the greatest anisotropy, with a strong tendency to vary in a plane oriented upwards at a 45° angle to the horizontal plane (see Fig. 9 ). In several cortical regions the principal directions of variability (along which the glyphs are elongated in Fig. 9
). In several cortical regions the principal directions of variability (along which the glyphs are elongated in Fig. 9 ) were approximately orthogonal to the primary gyral pattern. This directional trend was similar in some respects to the torquing, or petalia, which causes cortical regions in the right hemisphere to be situated slightly anterior to their counterparts on the left (Galaburda and Geschwind, 1981
) were approximately orthogonal to the primary gyral pattern. This directional trend was similar in some respects to the torquing, or petalia, which causes cortical regions in the right hemisphere to be situated slightly anterior to their counterparts on the left (Galaburda and Geschwind, 1981 ; Bilder et al., 1994
; Bilder et al., 1994 ). The region of highly anisotropic variability was strongly localized to the temporo-parietal cortex and did not extend anteriorly into the post-central and central gyri. A marked anatomical division occurred at the post-central gyrus, where variability was reduced and was spatially more isotropic. The component of variability normal to the average cortex was greatest at the temporal poles, where gyral patterns are relatively stable and variations in temporal lobe size may dominate. Importantly, this directional cortical variability is controlled by surface matching within the continuum-mechanical atlas, thereby allowing accurate maps of disease-related gray matter loss to be constructed (Figs 5,7
). The region of highly anisotropic variability was strongly localized to the temporo-parietal cortex and did not extend anteriorly into the post-central and central gyri. A marked anatomical division occurred at the post-central gyrus, where variability was reduced and was spatially more isotropic. The component of variability normal to the average cortex was greatest at the temporal poles, where gyral patterns are relatively stable and variations in temporal lobe size may dominate. Importantly, this directional cortical variability is controlled by surface matching within the continuum-mechanical atlas, thereby allowing accurate maps of disease-related gray matter loss to be constructed (Figs 5,7
 ).
).
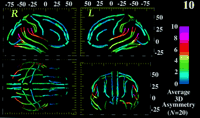
Cortical Pattern Asymmetry
Figure 10 illustrates the group average patterns of cortical asymmetry, highlighting regional trends. In a previous study we found Sylvian fissure asymmetry to be significantly greater in AD (P < 0.05) than in controls matched for age, gender and handedness (Thompson et al., 1998
illustrates the group average patterns of cortical asymmetry, highlighting regional trends. In a previous study we found Sylvian fissure asymmetry to be significantly greater in AD (P < 0.05) than in controls matched for age, gender and handedness (Thompson et al., 1998 ). Although these asymmetries are not apparent in every individual, a localized region can be clearly defined in which major asymmetrical trends are present (Fig. 10
). Although these asymmetries are not apparent in every individual, a localized region can be clearly defined in which major asymmetrical trends are present (Fig. 10 ). Severe asymmetry exhibited by the posterior Sylvian fissure (up to 10 mm) contrasted with negligible asymmetry in the frontal, parietal and occipital cortex (1–2 mm). The group average anatomy (Fig. 10
). Severe asymmetry exhibited by the posterior Sylvian fissure (up to 10 mm) contrasted with negligible asymmetry in the frontal, parietal and occipital cortex (1–2 mm). The group average anatomy (Fig. 10 ) shows the average Sylvian fissure terminating more posteriorly (P < 0.0002) and oriented more horizontally on the left than the right, corroborating postmortem measurements of the planum temporale (Geschwind and Levitsky, 1968
) shows the average Sylvian fissure terminating more posteriorly (P < 0.0002) and oriented more horizontally on the left than the right, corroborating postmortem measurements of the planum temporale (Geschwind and Levitsky, 1968 , Witelson and Kigar, 1992
, Witelson and Kigar, 1992 ; Galaburda, 1995
; Galaburda, 1995 ). The average right Sylvian fissure also shows an upward turn at its posterior limit (Fig. 10
). The average right Sylvian fissure also shows an upward turn at its posterior limit (Fig. 10 ) and is anterior to the posterior limit on the left (Thompson et al., 1998
) and is anterior to the posterior limit on the left (Thompson et al., 1998 ).
).
View larger version (34K):
[in this window]
[in a new window]
|
Figure 10. Population-based maps of cortical pattern asymmetry. Averaging of cortical patterns across subjects (n = 20, controls) reveals fundamental features in the profile of asymmetry across the normal human cortex. The marked brain asymmetry in the temporo-parietal cortex is clearly apparent, mapping its average magnitude in a population. Based on the average models for each cortical sulcus, asymmetry can be quantified locally, in 3D, revealing patterns not apparent in the cortical anatomy of an individual. Asymmetry is calculated based on 3D displacement maps, which subtract gyral models from mirror images of their counterparts in the opposite hemisphere.
|
|
Surprisingly, these average asymmetries continued anteriorly into the primary somatosensory cortex and posteriorly into the inferior temporal cortex (Fig. 10 ). In Figure 10
). In Figure 10 the right terminal rami of the superior and inferior temporal sulci, as well as the posterior ascending ramus of the Sylvian fissure, were up to 15 mm anterior to their counterparts on the left. This asymmetry continues into the post-central cortex, with the posterior bank of the post-central gyrus thrust forward by 8–9 mm on the right compared to the left (Fig. 10
the right terminal rami of the superior and inferior temporal sulci, as well as the posterior ascending ramus of the Sylvian fissure, were up to 15 mm anterior to their counterparts on the left. This asymmetry continues into the post-central cortex, with the posterior bank of the post-central gyrus thrust forward by 8–9 mm on the right compared to the left (Fig. 10 ). This asymmetry seems not to be explainable by the asymmetrical size of the left parietal operculum, which is larger in most cases on the left (Steinmetz et al., 1990
). This asymmetry seems not to be explainable by the asymmetrical size of the left parietal operculum, which is larger in most cases on the left (Steinmetz et al., 1990 ). The asymmetrical region also covers the territory occupied by the supramarginal gyrus, which surrounds the terminal ascending ramus of the Sylvian fissure in both brain hemispheres. The profile of asymmetry extends caudally across the planum parietale (Jäncke et al., 1994
). The asymmetrical region also covers the territory occupied by the supramarginal gyrus, which surrounds the terminal ascending ramus of the Sylvian fissure in both brain hemispheres. The profile of asymmetry extends caudally across the planum parietale (Jäncke et al., 1994 ) and across the lateral convexity of the cortex into the superior and inferior temporal gyri, where 3D variation reaches a peak of 14 mm and where several stereotyped variations in structure have been identified (Steinmetz et al., 1990
) and across the lateral convexity of the cortex into the superior and inferior temporal gyri, where 3D variation reaches a peak of 14 mm and where several stereotyped variations in structure have been identified (Steinmetz et al., 1990 ; Leonard, 1996
; Leonard, 1996 ).
).
 |
Discussion
|
|---|
By averaging cortical features in an AD population and matched elderly controls, striking profiles of gray matter loss, anatomical variation and cerebral asymmetry can be identified. Severe reductions in gray matter (up to 30% loss) were observed across the lateral temporal surfaces in the AD cohort. These deficits were also clearly found in the temporo-parietal cortices bilaterally. Patterns of left greater than right gray matter loss also became apparent, with severe gray matter loss observed bilaterally in the vicinity of Brodmann areas 9 and 46, regions of increased synaptic loss and ß-amyloid protein deposition (Clinton et al., 1994 ). There was also a comparative sparing of the superior post-central and central gyri and the occipital poles (0–5% loss, P < 0.05). This pattern is consistent with preservation of sensorimotor and visual function at this stage of the disease, at the same time as perfusion and metabolic deficits pervade in higher order association cortices.
). There was also a comparative sparing of the superior post-central and central gyri and the occipital poles (0–5% loss, P < 0.05). This pattern is consistent with preservation of sensorimotor and visual function at this stage of the disease, at the same time as perfusion and metabolic deficits pervade in higher order association cortices.
Hemispheric Differences
Interestingly, patterns of greater gray matter loss in the left hemisphere corroborate earlier reports (Loewenstein et al., 1989 ) of predominant left hemisphere metabolic dysfunction in mild to moderate AD, when cerebral glucose utilization is measured by positron emission tomography (PET). Structural, perfusion and metabolic studies suggest that the left hemisphere may be more susceptible to neuronal loss, instead of the alternative explanation that equivalent neuronal loss may result in greater functional deficits on one side, due to asymmetrical cortical organization. Greatest gray matter loss in the temporo-parietal cortex may underlie the prominent temporal-parietal hypometabolism that is consistently found at this stage of AD, often asymmetrically (Friedland and Luxenberg, 1988
) of predominant left hemisphere metabolic dysfunction in mild to moderate AD, when cerebral glucose utilization is measured by positron emission tomography (PET). Structural, perfusion and metabolic studies suggest that the left hemisphere may be more susceptible to neuronal loss, instead of the alternative explanation that equivalent neuronal loss may result in greater functional deficits on one side, due to asymmetrical cortical organization. Greatest gray matter loss in the temporo-parietal cortex may underlie the prominent temporal-parietal hypometabolism that is consistently found at this stage of AD, often asymmetrically (Friedland and Luxenberg, 1988 ; Johnson et al., 1998
; Johnson et al., 1998 ). Although the focus of this study was to determine patterns of gray matter loss in vivo, immunocytochemical studies have reported between 11 and 50% synaptic loss in the superior temporal and inferior parietal cortices, with a comparative sparing of occipital cortices (cf. Figs 5,7
). Although the focus of this study was to determine patterns of gray matter loss in vivo, immunocytochemical studies have reported between 11 and 50% synaptic loss in the superior temporal and inferior parietal cortices, with a comparative sparing of occipital cortices (cf. Figs 5,7
 ). Relatively greater atrophy is often reported in the temporal lobe relative to overall cerebral volume (Murphy et al., 1993
). Relatively greater atrophy is often reported in the temporal lobe relative to overall cerebral volume (Murphy et al., 1993 ). The early progression of AD pathology into the parietal and frontal association cortices suggests a degeneration of synaptically linked cortical pathways, and this pattern correlates with symptoms of memory impairment, aphasias, apraxias, personality changes and spatial deficits (Roberts et al., 1993
). The early progression of AD pathology into the parietal and frontal association cortices suggests a degeneration of synaptically linked cortical pathways, and this pattern correlates with symptoms of memory impairment, aphasias, apraxias, personality changes and spatial deficits (Roberts et al., 1993 ). Interestingly, gray matter loss at autopsy is predominantly cortical in Alzheimer's patients under 80 years of age (Hubbard and Anderson, 1981
). Interestingly, gray matter loss at autopsy is predominantly cortical in Alzheimer's patients under 80 years of age (Hubbard and Anderson, 1981 ), when volumes of subcortical nuclei are not significantly different between patients and controls (De La Monte, 1989
), when volumes of subcortical nuclei are not significantly different between patients and controls (De La Monte, 1989 ). Nonetheless, atrophy of the amygdala and basal nuclei (Cuénod et al., 1993
). Nonetheless, atrophy of the amygdala and basal nuclei (Cuénod et al., 1993 ) may ultimately be followed by alterations in thalamic nuclei (Jernigan et al., 1991
) may ultimately be followed by alterations in thalamic nuclei (Jernigan et al., 1991 ), induced perhaps by degeneration of their cortical projection areas.
), induced perhaps by degeneration of their cortical projection areas.
Profiles of Tissue Loss
While the lateral temporal and parietal cortices exhibit diffuse gray matter loss, some regions of the central and paracentral cortex appear to have several foci of average gray matter loss in territory that is otherwise comparatively spared (Fig. 7 ). Gray matter loss within a gyrus may be a multifocal process (as, for example, the discrete lesions in vascular dementia) or may occur rather uniformly within individual gyri. Clearly, some features occur at small spatial scales in both the statistical (P value) maps and the average loss maps. This multifocal effect does not appear to be attributable to sampling error in estimating the variance for the gray matter measure, as these variance values are spatially quite homogeneous. Structural and functional features with a spatial scale smaller than a gyrus may begin to be resolved if data from corresponding gyri are better aligned across subjects when averaging features from a population (Thompson et al., 2000a
). Gray matter loss within a gyrus may be a multifocal process (as, for example, the discrete lesions in vascular dementia) or may occur rather uniformly within individual gyri. Clearly, some features occur at small spatial scales in both the statistical (P value) maps and the average loss maps. This multifocal effect does not appear to be attributable to sampling error in estimating the variance for the gray matter measure, as these variance values are spatially quite homogeneous. Structural and functional features with a spatial scale smaller than a gyrus may begin to be resolved if data from corresponding gyri are better aligned across subjects when averaging features from a population (Thompson et al., 2000a ; Zeineh et al., 2000
; Zeineh et al., 2000 ). Conversely, gyral features may be blurred out (cf. Fig. 3
). Conversely, gyral features may be blurred out (cf. Fig. 3 ) when these correspondences are not taken into account (Evans et al., 1994
) when these correspondences are not taken into account (Evans et al., 1994 ). We did not hypothesize this multifocal effect in advance, so we did not test for its significance specifically. Longitudinal studies may allow us to better understand the scale and consistency of these localized changes over time and may reveal whether gray matter loss is an inherently diffuse or multifocal process within individual cortical gyri.
). We did not hypothesize this multifocal effect in advance, so we did not test for its significance specifically. Longitudinal studies may allow us to better understand the scale and consistency of these localized changes over time and may reveal whether gray matter loss is an inherently diffuse or multifocal process within individual cortical gyri.
Advantages of Gray Matter Maps
Cortical gray matter is lost in AD in a pattern that is temporally stereotyped and, initially, regionally specific. By resolving this pattern across the cortex, a detailed evaluation of degenerative change can be made in living populations. Conventional volumetric analysis of MRI data shows substantial overlap in both lobar volumes and gray matter measures between patients and controls, often because of difficulties in identifying equivalent areas of cortex. Overall structure volumes also display considerable variability. High dimensional registration (i.e. elastic matching) of cortical maps offers a solution to this difficulty, in that local measurements of gray matter can be calibrated against a local measure of tissue variance. Large differences in cortical organization are also readily accommodated.
Cortical Pattern Matching
The goal of the cortical matching procedure is to bring cortical regions into correspondence, so that data from corresponding regions can be averaged together across subjects. Without a procedure to align cortical structures, such as the one described in this paper, an averaging procedure applied voxel-by-voxel in stereotaxic space does not always average data from the same region of cortex and, in principle, data from the temporal cortex of some subjects could be averaged with data from the frontal cortex of other subjects. The gyral matching procedure alleviates this problem to a degree, although it does not solve it completely. Gyral matching does not guarantee that data from corresponding cytoarchitectonic regions will be averaged together. However, many functional regions of the cortex defined by PET and functional MRI (Watson et al., 1993 ), as well as many cyto-architectonic regions (Rademacher et al., 1993
), as well as many cyto-architectonic regions (Rademacher et al., 1993 ), bear a consistent relationship to macroanatomical landmarks of the gyral pattern. The degree to which cortical pattern matching reduces architectonic and functional variation can be evaluated by quantifying residual variability of functional or cellular landmarks after normalizing gross anatomical features (Rajkowska and Goldman-Rakic, 1995
), bear a consistent relationship to macroanatomical landmarks of the gyral pattern. The degree to which cortical pattern matching reduces architectonic and functional variation can be evaluated by quantifying residual variability of functional or cellular landmarks after normalizing gross anatomical features (Rajkowska and Goldman-Rakic, 1995 ; Van Essen and Drury, 1997
; Van Essen and Drury, 1997 ; Fox et al., 1999
; Fox et al., 1999 ; Geyer et al., 2000
; Geyer et al., 2000 ). Differences in the topological layout of architectonic regions within the cortical sheet ultimately preclude the mapping of discrete cortical regions from one subject to another, so an important intermediate goal has been to identify and match a comprehensive network of sulcal and gyral elements which are consistent in their incidence and topology across subjects (Ono et al., 1990
). Differences in the topological layout of architectonic regions within the cortical sheet ultimately preclude the mapping of discrete cortical regions from one subject to another, so an important intermediate goal has been to identify and match a comprehensive network of sulcal and gyral elements which are consistent in their incidence and topology across subjects (Ono et al., 1990 ; Rademacher et al., 1993
; Rademacher et al., 1993 ; Thompson et al., 1996a
; Thompson et al., 1996a , 1997
, 1997 ). While gyral matching substantially reduces the variability in cortical organization across subjects, in the future functional and architectonic landmarks may be definable in vivo that better guarantee matching of the cortical mantle from one subject to another in population studies (Dumoulin et al., 2000
). While gyral matching substantially reduces the variability in cortical organization across subjects, in the future functional and architectonic landmarks may be definable in vivo that better guarantee matching of the cortical mantle from one subject to another in population studies (Dumoulin et al., 2000 ).
).
At this stage, the pathological burden of AD may be greater in terms of functional deficits, and synaptic loss, in the heteromodal cortex than in the idiotypic cortex. In our prior studies AD patients exhibited significantly greater asymmetry and structural variability in the deep perisylvian cortex, relative to controls matched for age, gender, educational level and handedness (P < 0.05) (Thompson et al., 1998 ). Clear differences in both AD cortical variation and gray matter distribution suggest the need for disease-specific brain atlases that better reflect the disease-related anatomy of patients and calibrate individual loss against statistical data from normative populations.
). Clear differences in both AD cortical variation and gray matter distribution suggest the need for disease-specific brain atlases that better reflect the disease-related anatomy of patients and calibrate individual loss against statistical data from normative populations.
Emerging Patterns
In both groups anatomical features emerged that are not observed in individual representations due to their considerable variability. As shown in Figure 10 , the marked anatomical asymmetry in the posterior perisylvian cortex (Geschwind and Levitsky, 1968
, the marked anatomical asymmetry in the posterior perisylvian cortex (Geschwind and Levitsky, 1968 ) extends rostrally into the post-central cortex. The posterior bank of the post-central gyrus is thrust forward by 8–9 mm on the right compared with the left (Fig. 10
) extends rostrally into the post-central cortex. The posterior bank of the post-central gyrus is thrust forward by 8–9 mm on the right compared with the left (Fig. 10 ). This asymmetry extends caudally across the lateral convexity into the superior and inferior temporal cortex. As shown by averaging models of ventricular anatomy (Thompson et al., 2000d
). This asymmetry extends caudally across the lateral convexity into the superior and inferior temporal cortex. As shown by averaging models of ventricular anatomy (Thompson et al., 2000d ), this asymmetrical trend penetrates subcortically into the occipital horns of the lateral ventricles, but not into adjacent parieto-occipital and calcarine cortex (Thompson et al., 1998
), this asymmetrical trend penetrates subcortically into the occipital horns of the lateral ventricles, but not into adjacent parieto-occipital and calcarine cortex (Thompson et al., 1998 ). In contrast with existing brain atlases based on a single brain hemisphere (Talairach and Tournoux, 1988
). In contrast with existing brain atlases based on a single brain hemisphere (Talairach and Tournoux, 1988 ), population-based atlases encode information on asymmetry and its group variation, so that departures from normal patterns in individuals or groups can be identified (Thompson et al., 1997
), population-based atlases encode information on asymmetry and its group variation, so that departures from normal patterns in individuals or groups can be identified (Thompson et al., 1997 ; Thirion et al., 1998
; Thirion et al., 1998 ; Thompson and Toga, 1998
; Thompson and Toga, 1998 ; Cao and Worsley, 2000
; Cao and Worsley, 2000 ). There is a substantial literature on Sylvian fissure cortical surface asymmetries (Eberstaller, 1884
). There is a substantial literature on Sylvian fissure cortical surface asymmetries (Eberstaller, 1884 ; Cunningham, 1892
; Cunningham, 1892 ; Geschwind and Levitsky, 1968
; Geschwind and Levitsky, 1968 ; Davidson and Hugdahl, 1994
; Davidson and Hugdahl, 1994 ) and their relation to functional lateralization (Strauss et al., 1983
) and their relation to functional lateralization (Strauss et al., 1983 ), handedness (Witelson and Kigar, 1992
), handedness (Witelson and Kigar, 1992 ), language function (Davidson and Hugdahl, 1994
), language function (Davidson and Hugdahl, 1994 ), asymmetries of associated cytoarchitectonic fields (Galaburda and Geschwind, 1981
), asymmetries of associated cytoarchitectonic fields (Galaburda and Geschwind, 1981 ) and their thalamic projection areas (Eidelberg and Galaburda, 1982
) and their thalamic projection areas (Eidelberg and Galaburda, 1982 ), However, no prior reports have mapped the asymmetry profile across the cortex in three dimensions. These localized patterns of asymmetry in cortical morphology clearly have multiple determinants. We previously found Sylvian fissure asymmetry to be significantly greater in AD patients than in controls matched for age, gender, educational level and handedness (P < 0.05) (Thompson et al., 1998
), However, no prior reports have mapped the asymmetry profile across the cortex in three dimensions. These localized patterns of asymmetry in cortical morphology clearly have multiple determinants. We previously found Sylvian fissure asymmetry to be significantly greater in AD patients than in controls matched for age, gender, educational level and handedness (P < 0.05) (Thompson et al., 1998 ), suggesting that AD pathology asymmetrically disrupts the anatomy of the temporo-parietal cortex. The improved ability to localize asymmetries of cortical organization or tissue loss in a group atlas presents opportunities to analyze diseases with asymmetrical progression, including different stages of AD, and to map hypothesized alterations in cortical and hippocampal asymmetry in disease states such as schizophrenia (Falkai et al., 1992
), suggesting that AD pathology asymmetrically disrupts the anatomy of the temporo-parietal cortex. The improved ability to localize asymmetries of cortical organization or tissue loss in a group atlas presents opportunities to analyze diseases with asymmetrical progression, including different stages of AD, and to map hypothesized alterations in cortical and hippocampal asymmetry in disease states such as schizophrenia (Falkai et al., 1992 ; Kikinis et al., 1994
; Kikinis et al., 1994 ; Kulynych et al., 1996
; Kulynych et al., 1996 ; Csernansky et al., 1998
; Csernansky et al., 1998 ).
).
Population-based Brain Templates
From a practical standpoint, approaches for anatomical averaging also provide an average anatomical image template to represent a particular clinical group. In contrast to earlier studies, we matched cortical patterns across subjects to resolve fundamental anatomical features across a group. Similar approaches are under active development to create average brain representations for the macaque (Grenander and Miller, 1998 ) and for individual structures such as the corpus callosum (Gee et al., 1995
) and for individual structures such as the corpus callosum (Gee et al., 1995 ; Davatzikos, 1996
; Davatzikos, 1996 ), central sulcus (Manceaux-Demiau et al., 1998
), central sulcus (Manceaux-Demiau et al., 1998 ), cingulate and paracingulate sulci (Paus et al., 1996
), cingulate and paracingulate sulci (Paus et al., 1996 ), hippocampus (Haller et al., 1997
), hippocampus (Haller et al., 1997 ; Csernansky et al., 1998
; Csernansky et al., 1998 ; Joshi et al., 1998
; Joshi et al., 1998 ) and for transformed representations of the human and macaque cortex (Drury and Van Essen, 1997
) and for transformed representations of the human and macaque cortex (Drury and Van Essen, 1997 ; Grenander and Miller, 1998
; Grenander and Miller, 1998 ; Fischl et al., 1999
; Fischl et al., 1999 ). The resulting averages provide templates in which multimodality brain maps can be integrated (Mazziotta et al., 1995
). The resulting averages provide templates in which multimodality brain maps can be integrated (Mazziotta et al., 1995 ; Toga and Thompson, 1998
; Toga and Thompson, 1998 ). The probabil-istic information they contain can also guide Bayesian approaches for automatically identifying anatomical structures (Gee et al., 1995
). The probabil-istic information they contain can also guide Bayesian approaches for automatically identifying anatomical structures (Gee et al., 1995 ; Mangin et al., 1995
; Mangin et al., 1995 ; Royackkers et al., 1996
; Royackkers et al., 1996 ; Pitiot et al., 2000
; Pitiot et al., 2000 ). Finally, these probabilistic atlases can constrain the search space for activations in functional imaging experiments (Dinov et al., 2000
). Finally, these probabilistic atlases can constrain the search space for activations in functional imaging experiments (Dinov et al., 2000 ).
).
A group-specific atlas of the brain in early AD enables functional, metabolic and tissue distribution data to be analyzed in an anatomical framework that reflects AD morphology. The effects of morphological variation can also be controlled. However, the strategy described here is applicable, in principle, to any population. Since AD is a progressive disease, a homogeneous patient group was selected for this study, matched for age and educational level, at a stage in the disease when patients often present for initial evaluation and where MR, PET and SPECT scans may have maximal diagnostic value. By expanding the underlying patient database and stratifying the population according to different criteria, atlases to represent the more advanced stages of AD, or other clinically defined groups, could also be developed.
Longitudinal Studies
Longitudinal studies, in which a cohort of subjects is scanned repeatedly over time, show considerable promise in tracking the dynamics of normal aging and dementia. The mean rate of brain atrophy in AD, based on MRI measures of total cerebral volumes, was recently reported to be 2.4 ± 1.1% per year in AD, compared with 0.4 ± 0.5% per year in matched elderly controls (MMSE 19.6 ± 4.1 and 29.2 ± 1.0 at baseline, for patients and controls, respectively) (Fox et al., 2000 ). Higher rates of atrophy and tissue loss have been estimated for specific structures, including the hippocampus (Kaye et al., 1997
). Higher rates of atrophy and tissue loss have been estimated for specific structures, including the hippocampus (Kaye et al., 1997 ; Jack et al., 1998
; Jack et al., 1998 ; Laakso et al., 2000
; Laakso et al., 2000 ). Four-dimensional maps of degenerative rates may also be derived by computing a deformation field that elastically transforms a subject's anatomy from its earlier configuration to its shape in a later scan (Fox et al., 1996
). Four-dimensional maps of degenerative rates may also be derived by computing a deformation field that elastically transforms a subject's anatomy from its earlier configuration to its shape in a later scan (Fox et al., 1996 , 2000
, 2000 ; Thompson et al., 2000b
; Thompson et al., 2000b ,d
,d ). We are currently extending the mapping approach described here to store detailed population-based maps of degenerative rates across time and explore linkages between these maps and cognitive variables (Thompson et al., 2000d
). We are currently extending the mapping approach described here to store detailed population-based maps of degenerative rates across time and explore linkages between these maps and cognitive variables (Thompson et al., 2000d ), as well as therapeutic and genetic factors [e.g. ApoE genotype (Small et al., 2000)].
), as well as therapeutic and genetic factors [e.g. ApoE genotype (Small et al., 2000)].
Accurate mapping of gray matter changes in a living population with AD holds significant promise for genetic, longitudinal and interventional studies of dementia. In any study where staging of the disease is required, the ability to calibrate gray matter integrity against a reference population is paramount. The patient cohort on which our atlas is based is being expanded to accommodate groups at different stages of dementia. By following the same patients longitudinally (Thompson et al., 2000b ), statistical maps of gray matter loss at multiple time points will ultimately provide a dynamic frame-work to help understand the progression of the disease and to gauge therapeutic, disease-modifying response in an individual or clinically defined group.
), statistical maps of gray matter loss at multiple time points will ultimately provide a dynamic frame-work to help understand the progression of the disease and to gauge therapeutic, disease-modifying response in an individual or clinically defined group.
 |
Notes
|
|---|
This work was supported by a Human Brain Project grant to the International Consortium for Brain Mapping, funded jointly by NIMH and NIDA (P20 MH/DA52176), by a P41 Resource Grant from the NCRR (RR13642), by NINCDS grant K08-NS01646, NIA grant K08-AG100784 and research grants from the National Library of Medicine (LM/MH05639), the National Science Foundation (BIR 93-22434), the NCRR (RR05956) and NINCDS/NIMH (NS38753).
Address correspondence to Paul Thompson, Room 4238, Reed Neurological Research Center, Laboratory of Neuro Imaging, Department of Neurology, UCLA School of Medicine, 710 Westwood Plaza, Los Angeles, CA 90095–1769, USA. Email: thompson@loni.ucla.edu
 |
Appendix
|
|---|
Constructing Variability Maps
The imposition of a standard surface grid on each subject's cortex makes it easier to compare anatomical models from multiple subjects. By averaging nodes with the same grid coordinates across subjects, an average surface is produced for each group. The mean surface acts as a reference surface, relative to which deviations (displacements) in the other surfaces are measured. Information on subjects' individual differences is then stored as a vector-valued displacement map, indicating how a subject deviates locally from the average anatomy. Color maps that illustrate the spatial variability of anatomy in a group were computed as by Thompson and co-workers (Thompson et al., 1996b ). Briefly, for a group of n subjects, the variability in spatial position for points ri(u,v) internal to a particular anatomical surface is computed, based on the maps, as a scalar variance function:
). Briefly, for a group of n subjects, the variability in spatial position for points ri(u,v) internal to a particular anatomical surface is computed, based on the maps, as a scalar variance function:
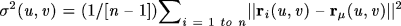 | (1) |
defined at each mesh node (u,v), where rµ(u,v) is the average surface. The square root of this function gives the standard deviation in stereotaxic position as a 3D r.m.s. distance for each internal surface point. The appropriate numerical value, at each grid point, is given by the root mean square magnitude of the 3D displacement vectors assigned to that point, in the n surface maps from the individual to average. The variability measure is visualized using a color code to illustrate the profile of variability across the anatomy.
Elastic Matching of Gyral Patterns using Flow Fields
Differences in cortical patterns between any pair of subjects were determined by deforming one cortical model to match the other. This procedure has been covered in detail by Thompson and co-workers (Thompson et al., 2000a ) and is summarized here for completeness. Since each cortical model is obtained by deforming a spherical surface into the shape of the cortex, gyral features can be mapped back onto a sphere and, subsequently, to a plane (Fig. 2
) and is summarized here for completeness. Since each cortical model is obtained by deforming a spherical surface into the shape of the cortex, gyral features can be mapped back onto a sphere and, subsequently, to a plane (Fig. 2 ). This simplifies computation of anatomical correspondences. Anatomical correspondences can therefore be computed by defining a flow field in the flat, 2D parameter space that matches gyral features from one subject to another (Figs 2, 3
). This simplifies computation of anatomical correspondences. Anatomical correspondences can therefore be computed by defining a flow field in the flat, 2D parameter space that matches gyral features from one subject to another (Figs 2, 3
 ) (Davatzikos et al., 1996
) (Davatzikos et al., 1996 ; Thompson et al., 1996a
; Thompson et al., 1996a , 1997
, 1997 , 2000d
, 2000d ; Fischl et al., 1999
; Fischl et al., 1999 ). The flow is given by the solution to a curve-driven warp in the flat parametric space of the cortex (Thompson et al., 1996, 1998
). The flow is given by the solution to a curve-driven warp in the flat parametric space of the cortex (Thompson et al., 1996, 1998 , 2000). The flow behavior is modeled using equations derived from continuum mechanics and these equations are governed by the Cauchy–Navier differential operator L = µ
, 2000). The flow behavior is modeled using equations derived from continuum mechanics and these equations are governed by the Cauchy–Navier differential operator L = µ 2 + (
2 + ( + µ)
+ µ) (
( T•) (Davatzikos et al., 1996
T•) (Davatzikos et al., 1996 ; Thompson et al., 1996, 1998
; Thompson et al., 1996, 1998 , 2000d
, 2000d ; Grenander and Miller, 1998
; Grenander and Miller, 1998 ).
).
Technical Details
Specifically, for points r = (r,s) in the cortical parameter space  = [0,2
= [0,2 ) x [0,
) x [0, ), a system of simultaneous partial differential equations can be written for the flow field u (r):
), a system of simultaneous partial differential equations can be written for the flow field u (r):
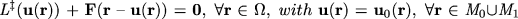 | (2) |
Here M0, M1 are sets of points and (sulcal or gyral) curves where displacement vectors u(r) = u0(r) matching the corresponding anatomy across subjects are known. The flow behavior is governed by the Cauchy–Navier differential operator L = µ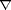 2 + (
2 + (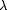 + µ)
+ µ)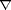 (
(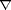 T•) with body force F (Thompson et al., 1996, 1998
T•) with body force F (Thompson et al., 1996, 1998 , 2000; Grenander and Miller, 1998
, 2000; Grenander and Miller, 1998 ). In solving this governing equation matching sulcal networks across subjects, dependencies between the metric tensors of the surface parameterizations and the matching field are eliminated with an approach known as covariant regularization, which uses generalized coordinates and correction terms known as Christoffel symbols (Thompson and Toga, 2000a,d). Because of the intrinsic curvature of the cortex, this means that the ‘covariant form’ L
). In solving this governing equation matching sulcal networks across subjects, dependencies between the metric tensors of the surface parameterizations and the matching field are eliminated with an approach known as covariant regularization, which uses generalized coordinates and correction terms known as Christoffel symbols (Thompson and Toga, 2000a,d). Because of the intrinsic curvature of the cortex, this means that the ‘covariant form’ L of the differential operator L is used when solving these equations (Thompson and Toga, 1998
of the differential operator L is used when solving these equations (Thompson and Toga, 1998 ; Thompson et al., 2000d
; Thompson et al., 2000d ). This adjustment also makes sure that cortical surfaces are matched in a way that is actually independent of the way the surfaces are flattened; in other words, the matching procedure is parameterization-invariant. In the partial differential equations (2)
). This adjustment also makes sure that cortical surfaces are matched in a way that is actually independent of the way the surfaces are flattened; in other words, the matching procedure is parameterization-invariant. In the partial differential equations (2) we replace L by the covariant differential operator L
we replace L by the covariant differential operator L . In L
. In L all L value partial derivatives are replaced by covariant derivatives. These covariant derivatives are defined with respect to the metric tensor of the surface domain where calculations are performed. The covariant derivative of a (contravariant) vector field, ui(x), is defined as ui,k =
all L value partial derivatives are replaced by covariant derivatives. These covariant derivatives are defined with respect to the metric tensor of the surface domain where calculations are performed. The covariant derivative of a (contravariant) vector field, ui(x), is defined as ui,k =  uj/
uj/ xk +
xk +  jik ui, where the Christoffel symbols of the second kind,
jik ui, where the Christoffel symbols of the second kind, 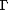 jik, are computed from derivatives of the metric tensor components gjk(x):
jik, are computed from derivatives of the metric tensor components gjk(x):
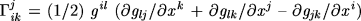 | (3) |
These correction terms are then used in the elastic transformation used to match one cortex with another.
Finally, because 3D cortical positions are encoded in color on the flat maps, the surface matching transformation is recovered in 3D as a mapping that drives one cortex onto another. A color code (Fig. 2e ) representing 3D cortical point locations in an individual subject is convected along with the flow that drives the sulcal pattern into the average configuration for the group (Fig. 2f
) representing 3D cortical point locations in an individual subject is convected along with the flow that drives the sulcal pattern into the average configuration for the group (Fig. 2f ). Once this is done in all subjects at a particular location in the flat map (Fig. 2f
). Once this is done in all subjects at a particular location in the flat map (Fig. 2f ), points on each individual's cortex are recovered that have the same relative location to the primary folding pattern in all subjects. Averaging of these corresponding points results in a crisp average cortex (Fig. 3
), points on each individual's cortex are recovered that have the same relative location to the primary folding pattern in all subjects. Averaging of these corresponding points results in a crisp average cortex (Fig. 3 , bottom row). The corresponding 3D displacement is recovered between the cortical models. This displacement matches a large network of sulcal features and thus is a valid encoding of gyral pattern differences.
, bottom row). The corresponding 3D displacement is recovered between the cortical models. This displacement matches a large network of sulcal features and thus is a valid encoding of gyral pattern differences.
View larger version (89K):
[in this window]
[in a new window]
|
Figure 6. 3D cortical variability (n = 26, AD patients). The profile of variability across the cortex is shown, after differences in brain orientation and size are removed. The following views are shown: oblique frontal, frontal, right, left, top, bottom. Extreme variability in the posterior perisylvian zones and superior frontal association cortex (12–14 mm, red) contrasts with the comparative invariance of the primary sensory, motor and orbitofrontal cortex (2–5 mm, blue). Models are orthographically projected onto a coordinate grid to facilitate comparisons with data from functional and metabolic studies (Mega et al., 2000).
|
|
 |
Footnotes
|
|---|
1 These additional boundaries included: (i) the posterior-medial limit of the occipital lobe in each hemisphere, between the parieto-occipital and posterior calcarine sulci; (ii) the inferior limit of the lingual gyrus at the medial wall of each brain hemisphere, from the posterior calcarine sulcus to the splenium of the corpus callosum; (iii) the superior-medial boundary of the parietal lobe, from the parieto-occipital to the central sulcus; (iv) the anterior boundary of the frontal lobes, from the superior-medial limit of the central sulcus to the antero-medial tip of the superior rostral sulcus; (v) the inferior boundary of the frontal lobes, from the superior rostral sulcus posteriorly and inferiorly along the rhinal gyri to the rostral tip of the anterior commissure. 
2 Significance levels. If there had been no pre-existing hypothesis on the localization of significant gray matter loss, which was expected in the temporal and temporo-parietal cortex, a correction for multiple comparisons can be made. The significance threshold can be set at a level derived from the effective number of resolution elements in the statistical field (RESELs) (Worsley, 1994 ). This corrected P value depends on the smoothness tensor of the residuals of the statistical model, which can also be estimated from the surface data, using an approach known as statistical flattening (Worsley et al., 1999
). This corrected P value depends on the smoothness tensor of the residuals of the statistical model, which can also be estimated from the surface data, using an approach known as statistical flattening (Worsley et al., 1999 ; Thompson et al., 2000d
; Thompson et al., 2000d ).
). 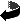
 |
References
|
|---|
Bilder RM, Wu H, Chakos MH, Bogerts B, Pollack S, Aronowitz J, Ashtari M, Degreef G, Kane JM, Lieberman JA (1994) Cerebral morphometry and clozapine treatment in schizophrenia. J Clin Psychiat 55 (suppl B):53–56.
Brodmann K (1909) Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues, Barth, Leipzig. In: Some papers on the cerebral cortex. [Translated as: Brodmann K (1960) On the comparative localization of the cortex, pp. 201–230. Springfield, IL: Thomas.]
Cao J, Worsley KJ (2000) The geometry of the Hotelling's T-squared random field with applications to the detection of shape changes. Ann Statist (in press).
Clinton J, Blackman SEA, Royston MC, Robert GW (1994) Differential synaptic loss in the cortex in AD: a study using archival material. NeuroReport 5:497–500.[Medline]
Collins DL, Le Goualher G, Venugopal R, Caramanos A, Evans AC, Barillot C (1996) Cortical constraints for non-linear cortical registration, In: Visualization in biomedical computing, Hamburg, Germany, Sept. 1996, Lecture notes in computer science (Höhne KH, Kikinis R, eds), 1131, pp. 307–316. Berlin: Springer Verlag.
Cook MJ, Free SL, Fish DR, Shorvon SD, Straughan K, Stevens JM (1994) Analysis of cortical patterns, In: Magnetic resonance scanning and epilepsy (Shorvon SD, ed), pp. 263–274. New York: Plenum.
Courchesne E (1997) Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Curr Opin Neurobiol 7:269–278.[Medline]
Csernansky JG, Joshi S, Wang L, Haller JW, Gado M, Miller JP, Grenander U, Miller MI (1998) Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proc Natl Acad Sci USA 95:11406–11411.[Abstract/Full Text]
Cuénod CA, Denys A, Michot JL, Jehenson P, Forette F, Kaplan D, Syrota A, Boller F (1993) Amygdala atrophy in AD: an in vivo magnetic resonance study. Arch Neurol 50:941–945.[Medline]
Cummings J, Benson DF, LoVerme S (1980) Reversible dementia. Illustrative cases, definition, and review. J Am Med Assoc 243: 2434–2439.
Cunningham DJ (1892) Contribution to the surface anatomy of the cerebral hemispheres. Cunningham Memoirs (R. Irish Acad.) 7:372.
Davatzikos C (1996) Spatial normalization of 3D brain images using deformable models. J. Comput. Assist Tomogr 20:656–665.[Medline]
Davatzikos C, Vaillant M, Resnick SM, Prince JL, Letovsky S, Bryan RN (1996) A computerized approach for morphological analysis of the corpus callosum. J. Comput. Assist Tomogr 20:88–97.[Medline]
Davidson RJ, Hugdahl K (1994) Brain asymmetry. Cambridge, MA: MIT Press.
De La Monte SM (1989) Quantification of cerebral atrophy in pre-clinical and end-stage AD. Ann Neurol 25:450–459.[Medline]
Dinov ID,Mega MS, Thompson PM, Lee L, Woods RP, Holmes CJ, Sumners DW, Toga AW (2000) Analyzing functional brain images in a probabilistic atlas: a validation of sub-volume thresholding. J Comput Assist Tomogr 24:128–138.[Medline]
Drury HA, Van Essen DC (1997) Analysis of functional specialization in human cerebral cortex using the Visible Man surface based atlas. Hum Brain Map 5:233–237.
Drury HA, Van Essen DC, Joshi SC, Miller MI (1996) Analysis and comparison of areal partitioning schemes using two-dimensional fluid deformations. NeuroImage 3:S130.
Dumoulin SO, Bittar RG, Kabani NJ, Baker CL Jr, Le Goualher G, Pike B, Evans AC (2000) A new anatomical landmark for reliable identification of human area V5/MT: a quantitative analysis of sulcal patterning. Cereb Cortex 10:454–463.[Abstract/Full Text]
Eberstaller O (1884) Zür Oberflachen Anatomie der Grosshirn Hemisphaeren. Wien Med Bl. 7:479,642,644.
Eidelberg D, Galaburda AM (1982) Symmetry and asymmetry in the human posterior thalamus: I. Cytoarchitectonic analysis in normal persons. Arch Neurol 39:325–332.[Medline]
Evans AC, Collins DL, Neelin P, MacDonald D, Kamber M, Marrett TS (1994) Three-dimensional correlative imaging: applications in human brain mapping. In: Functional neuroimaging: technical foundations (Thatcher RW, Hallett M, Zeffiro T, John ER, Huerta M, eds), pp. 145–162.
Falkai P, Bogerts B, Greve B, Pfeiffer U, Machus B, Folsch-Reetz B, Majtenyi C, Ovary I (1992) Loss of Sylvian fissure asymmetry in schizophrenia. A quantitative post mortem study. Schizophr Res 7:23–32.[Medline]
Filipek PA, Kennedy DN, Caviness VS Jr, Rossnick SL, Spraggins TA, Starewicz PM (1989) Magnetic resonance imaging-based brain morphometry: development and application to normal subjects. Ann Neurol 25:61–67.[Medline]
Fischl B, Sereno MI, Tootell RBH, Dale AM (1999) High-resolution inter-subject averaging and a coordinate system for the cortical surface. Hum Brain Map 8:272–284.
Folstein MF, Folstein SE, McHugh PR (1975) ‘Mini mental state’: a practical method of grading the cognitive state of patients for the clinician. J Psychiat Res 12:189–198.[Medline]
Fox NC, Freeborough PA, Rossor MN (1996) Visualisation and quantification of rates of atrophy in AD. Lancet 348:94–97.[Medline]
Fox NC, Cousens S, Scahill R, Harvey RJ, Rossor MN (2000) Using serial registered brain magnetic resonance imaging to measure disease progression in Alzheimer disease: power calculations and estimates of sample size to detect treatment effects. Arch Neurol 57:339–344.[Medline]
Fox PT, Huang AY, Parsons LM, Xiong JH, Rainey L, Lancaster JL (1999) Functional volumes modeling: scaling for group size in averaged images. Hum Brain Map 8:143–150.
Friedland RP, Luxenberg J (1988) Neuroimaging and dementia. In: Clinical neuroimaging: frontiers in clinical neuroscience, Vol. 4 (Theodore WH, ed), pp. 139–163. New York: Allan Liss.
Galaburda AM (1995) Anatomic basis of cerebral dominance. In: Brain asymmetry, (Davidson RJ, Hugdahl K, eds), pp. 51–73. Cambridge, MA: MIT Press,.
Galaburda AM, Geschwind N (1981) Anatomical asymmetries in the adult and developing brain and their implications for function. Adv Pediatr 28:271–292.[Medline]
Ge Y, Fitzpatrick JM, Kessler RM, Jeske-Janicka M (1995) Intersubject brain image registration using both cortical and subcortical landmarks. SPIE Image Process 2434:81–95.
Gee JC, LeBriquer L, Barillot C, Haynor DR, Bajcsy R (1995) Bayesian approach to the brain image matching problem, Institute for Research in Cognitive Science Technical Report 95-08, April 1995.
Geschwind N, Levitsky W (1968) Human brain: Left-right asymmetries in temporal speech region. Science 161:186.[Medline]
Geyer S, Schormann T, Mohlberg H, Zilles K (2000) Areas 3a, 3b, and 1 of human primary somatosensory cortex. NeuroImage 11:684–696.[Medline]
Giedd JN, Castellanos FX, Casey BJ, Kozuch P, King AC, Hamburger SD, Rapaport JL (1994) Quantitative morphology of the corpus callosum in attention deficit hyperactivity disorder. Am J Psychiat 151:665–669.[Medline]
Grenander U, Miller MI (1998) Computational anatomy: an emerging discipline, Technical Report, Department of Mathematics, Brown University.
Haller JW, Banerjee A, Christensen GE, Gado M, Joshi S, Miller MI, Sheline Y, Vannier MW, Csernansky JG (1997) Three-dimensional hippocampal MR morphometry with high-dimensional transformation of a neuroanatomic atlas. Radiology 202:504–510.[Abstract]
Holmes CJ, MacDonald D, Sled JG, Toga AW, Evans AC (1996) Cortical peeling: CSF/grey/white matter boundaries visualized by nesting isosurfaces. Proc Visualizat Biomed Comput 4:99–104.
Hubbard BM, Anderson JM (1981) A quantitative study of cerebral atrophy in old age and senile dementia. J Neurol Sci 50:135–145.[Medline]
Jack CR Jr, Petersen RC, Xu Y, O'Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Kokmen E (1998) Rate of medial temporal lobe atrophy in typical aging and Alzheimer's disease. Neurology 51:993–999.[Abstract]
Jäncke L, Schlaug G, Huang Y, Steinmetz H (1994) Asymmetry of the planum parietale. NeuroReport 5:1161–1163.[Medline]
Jernigan TL, Salmon D, Butter N, et al. (1991) Cerebral structure on MRI, Part II: specific changes in Alzheimer's and Huntington's diseases. Biol Psychiat 29:68–81.[Medline]
Johnson KA, Jones K, Holman BL, Becker JA, Spiers PA, Satlin A, Albert MS (1998) Preclinical prediction of Alzheimer's disease using SPECT. Neurology 50:1563–1571.[Abstract]
Joshi S, Miller MI, Grenander U (1998) On the geometry and shape of brain sub-manifolds. Int J Pattern Recogn Artif Intell 11:1317–1343.
Kaye JA, Swihart T, Howieson D, Dame A, Moore MM, Karnos T, Camicioli R, Ball M, Oken B, Sexton G (1997) Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology 48:1297–1304.[Abstract]
Kennedy DN, Lange N, Makris N, Bates J, Meyer J, Caviness VS Jr (1998) Gyri of the human neocortex: an MRI-based analysis of volume and variance. Cereb Cortex 8:372–384.[Abstract]
Kikinis R, Shenton ME, Gerig G, Hokama H, Haimson J, O'Donnell BF, Wible CG, McCarley RW, Jolesz FA (1994) Temporal lobe sulco-gyral pattern anomalies in schizophrenia: an in vivo MR three-dimensional surface rendering study. Neuroscience Lett 182:7–12.
Kulynych JJ, Vladar K, Jones DW, Weinberger DR (1996) Superior temporal gyrus volume in schizophrenia: a study using MRI morphometry assisted by surface rendering. Am J Psychiat 153:50–56.[Medline]
Laakso MP, Lehtovirta M, Partanen K, Riekkinen PJ, Soininen H (2000) Hippocampus in AD: a 3-year follow-up MRI study. Biol Psychiat 47:557–561.[Medline]
Le Goualher G, Barillot C, Bizais Y, Scarabin J-M, (1996) 3D segmentation of cortical sulci using active models. SPIE Med Imaging 2710, 254–263.
Leonard CM (1996) Structural variation in the developing and mature cerebral cortex: noise or signal? In: Developmental neuroimaging: mapping the development of brain and behavior (Thatcher RW, Reid Lyon G, Rumsey J, Krasnegor N, eds) pp. 207–231. Academic Press.
Loewenstein DA, Barker WW, Chang JY, Apicella A, Yoshii F, Kothari P, Levin B, Duara R (1989) Predominant left hemisphere metabolic dysfunction in dementia. Arch. Neurol 46:146–152.[Medline]
MacDonald D (1998) A method for identifying geometrically simple surfaces from three dimensional images, PhD Thesis, McGill University, Canada.
MacDonald D, Avis D, Evans AC (1993) Automatic parameterization of human cortical surfaces. In Annual Symposium on Information Processing in Medical Imaging (IPMI).
MacDonald D, Avis D, Evans AC (1994) Multiple surface identification and matching in magnetic resonance imaging. Proc SPIE 2359:160–169.
MacDonald D, Venugopal R, Caramanos Z, Petrides M, Avis D, Evans AC (1997) A surface-based 2D sulcal atlas. NeuroImage 5:S414.
Malobabic S, Marinkovic R, Lesic A, Draganic S, Duranovic S, Sojic M (1993) Morphologic asymmetry of the frontal lobe of the cerebral hemisphere in man. Med Pregl 46:401–405.[Medline]
Manceaux-Demiau A, Bryan RN, Davatzikos C (1998) A probabilistic ribbon model for shape analysis of the cerebral sulci: application to the central sulcus. J Comput Assist Tomogr 22:962–971.[Medline]
Mangin J-F, Frouin V, Bloch I, Regis J, López–Krahe J (1995) From 3D magnetic resonance images to structural representations of the cortex topography using topology-preserving deformations. J Math Imaging Vision 5: 297–318.
Mazziotta JC, Toga AW, Evans AC, Fox P, Lancaster J (1995) A probabilistic atlas of the human brain: theory and rationale for its development. NeuroImage 2:89–101.[Medline]
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadian EM (1984) Clinical diagnosis of AD: report of the NINCDS-ARDRA Work Group under the Auspices of the Health and Human Services Task Force on Alzheimer's Disease. Neurology 34:939–944.[Abstract]
Mega MS, Chen S, Thompson PM, Woods RP, Karaca TJ, Tiwari A, Vinters H, Small GW, Toga AW (1997a) Mapping pathology to metabolism: coregistration of stained whole brain sections to PET in AD. NeuroImage 5:147–153.[Medline]
Mega MS, Chu T, Thompson PM, Mazziotta JC, Burt J, Aron J, Ghasri P, Chen S, Lim J, Cole GM, Toga AW (1997b) [18F]-Fluorodeoxyglucose positron emission tomography (FDG-PET) corrected with synaptophysin density is inversely related to beta-amyloid burden in AD. Ann Neurol 42:M23.
Mega MS, Thompson PM, Cummings JL, Back CL, Xu LQ, Zohoori S, Goldkorn A, Moussai J, Fairbanks L, Small GW, Toga AW (1998) Sulcal variability in the Alzheimer's brain: correlations with cognition. Neurology, 50:145–151.[Abstract]
Mega MS, Thompson PM, Toga AW, Cummings JL (2000a) Brain mapping in dementia. In: Brain mapping: the disorders (Toga AW, Mazziotta JC, eds). Academic Press (in press).
Mega MS, Thompson PM, Dinov ID, Toga AW, Cummings JL (2000b) The UCLA Alzheimer Brain Atlas Project: structural and functional applications. In: Proceedings of the 2000 World Alzheimer's Congress, Washington, DC.
Missir O, Dutheil-Desclercs C, Meder JF, Musolino A, Fredy D (1989) Central sulcus patterns at MRI. J Neuroradiol 16:133–144.[Medline]
Murphy DGM, DeCarli CD, Daly E, Gillette JA, McIntosh AR, Haxby JV, Teichberg D, Schapiro MB, Rapoport SI, Horwitz B (1993) Volumetric magnetic resonance imaging in men with dementia of the Alzheimer type: correlations with disease severity. Biol Psychiat 34:612–621.[Medline]
Novikov II, Podcherednik TN (1992) Variability of the gyri and sulci of the temporal lobe of the human brain. Zh Nevropatol Psikhiatr S S Korsakova 92:102–105.
Ono M, Kubik S, Abernathey CD (1990) Atlas of the cerebral sulci. Stuttgart: Thieme.
Paus T, Tomaioulo F, Otaky N, MacDonald D, Petrides M, Atlas J, Morris R, Evans AC (1996) Human cingulate and paracingulate sulci: pattern, variability, asymmetry and probabilistic map. Cereb Cortex 6:207–214.[Abstract]
Pitiot A, Thompson PM, Toga AW (2000) Spatially and temporally adaptive elastic template matching. IEEE Trans Pattern Anal Machine Intell (in press).
Rademacher J, Caviness VS Jr, Steinmetz H, Galaburda AM (1993) Topographical variation of the human primary cortices: implications for neuroimaging, brain mapping, and neurobiology. Cereb Cortex 3:313–329.[Abstract]
Rajkowska G, Goldman-Rakic P (1995) Cytoarchitectonic definition of pre-frontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach coordinate system. Cereb Cortex 5:323–337.[Abstract]
Roberts GW, Leigh PN, Weinberger DR (1993) Neuropsychiatric disorders, Ch 2(1). Gower Medical Publishers.
Royackkers N, Desvignes M, Revenu M (1996) Construction automatique d'un atlas adaptatif des sillons corticaux, ORASIS 96, Clermont-Ferrand, pp. 187–192.
Sled JG, Zijdenbos AP, Evans AC (1998) A non-parametric method for automatic correction of intensity non-uniformity in MRI data. IEEE Trans Med Imaging 17:87–97.[Medline]
Sobire G, Goutieres F, Tardieu M, Landrieu P, Aicardi J (1995) Extensive macrogyri or no visible gyri: distinct clinical, electroencephalographic, and genetic features according to different imaging patterns. Neurology 45:1105–1111.[Abstract]
Sowell ER, Thompson PM, Holmes CJ, Batth R, Trauner DA, Jernigan TL, Toga AW (1999a) Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. NeuroImage 9:587–597.[Medline]
Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW (1999b) Progression of structural changes in the human brain during the first three decades of life: in vivo evidence for post-adolescent frontal and striatal maturation. Nature Neurosci 2:859–861.[Medline]
Steinmetz H, Furst G, Freund H-J (1989) Cerebral cortical localization: application and validation of the proportional grid system in MR imaging. J Comput Assist Tomogr 13:10–19.[Medline]
Steinmetz H, Furst G, Freund H-J (1990) Variation of perisylvian and calcarine anatomic landmarks within stereotaxic proportional coordinates. Am J Neuroradiol 11:1123–1130.[Abstract]
Strauss E, Kosaka B, Wada J (1983) The neurobiological basis of lateralized cerebral function. A review. Hum Neurobiol 2:115–127.[Medline]
Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain. New York: Thieme.
Thirion J-P, Prima S, Subsol S (1998) Statistical analysis of dissymmetry in volumetric medical images. Med Image Anal (in press).
Thompson PM, Toga AW (1997) Detection, visualization and animation of abnormal anatomic structure with a deformable probabilistic brain atlas based on random vector field transformations. Med Image Anal 1:271–294.[Medline]
Thompson PM, Toga AW (1998) Anatomically-driven strategies for high-dimensional brain image warping and pathology detection. In: Brain warping (Toga AW, ed), pp. 311–336. Academic Press.
Thompson PM, Schwartz C, Toga AW (1996a) High-resolution random mesh algorithms for creating a probabilistic 3D surface atlas of the human brain. NeuroImage 3:19–34.[Medline]
Thompson PM, Schwartz C, Lin RT, Khan AA, Toga AW (1996b) 3D statistical analysis of sulcal variability in the human brain. J Neurosci 16:4261–4274.[Abstract/Full Text]
Thompson PM, MacDonald D, Mega MS, Holmes CJ, Evans AC, Toga AW (1997) Detection and mapping of abnormal brain structure with a probabilistic atlas of cortical surfaces. J Comp Assist Tomogr 21:567–581.
Thompson PM, Moussai J, Khan AA, Zohoori S, Goldkorn A, Mega MS, Small GW, Cummings JL, Toga AW (1998) Cortical variability and asymmetry in normal aging and Alzheimer's Disease. Cereb Cortex 8:492–509.[Abstract]
Thompson PM, Woods RP, Mega MS, Toga AW (2000a) Mathematical/ computational challenges in creating population-based brain atlases. Hum Brain Map 9:81–92.
Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW (2000b) Growth patterns in the developing human brain detected using continuum-mechanical tensor mapping. Nature, 404:190–193.[Medline]
Thompson PM, Mega MS, Cummings JL, Toga AW (2000c) Detecting dynamic (4D) profiles of degenerative rates in AD patients, using tensor mapping and a population-based brain atlas. Proc Soc Neurosci.
Thompson PM, Mega MS, Narr KL, Sowell ER, Blanton RE, Toga AW (2000d) Brain image analysis and atlas construction. In: SPIE Handbook on Medical Image Analysis (Fitzpatrick M, ed). Society of Photo-Optical Instrumentation Engineers (SPIE) Press.
Toga AW, Thompson PM (1998) Multimodal brain atlases. In: Advances in biomedical image databases (Wong S, ed), pp.53–88. Kluwer Academic Press.
Van Essen DC, Drury HA (1997) Structural and functional analyses of human cerebral cortex using a surface-based atlas. J Neurosci 17:7079–7102.[Abstract/Full Text]
Watson JD, Myers R, Frackowiak RS, Hajnal JV, Woods RP, Mazziotta JC, Shipp S, Zeki S (1993) Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb Cortex 3:79–94.[Abstract]
Witelson SF, Kigar DL (1992) Sylvian fissure morphology and asymmetry in men and women: bilateral differences in relation to handedness in men. J Comp Neurol 323:326–340.[Medline]
Woods RP, Mazziotta JC, Cherry SR (1993) MRI-PET pegistration with automated algorithm. J Comput Assist Tomogr 17:536–546.[Medline]
Woods RP, Grafton ST, Watson JDG, Sicotte NL, Mazziotta JC. (1998) Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr 22:153–165.[Medline]
Worsley KJ (1994) Local maxima and the expected Euler characteristic of excursion sets of chi-squared, F and t fields. Adv Appl Probab 26:13–42.
Worsley KJ, Andermann M, Koulis T, MacDonald D, Evans AC (1999) Detecting changes in non-isotropic images. Hum Brain Map 8:98–101.
Zeineh MM, Thompson PM, Engel SA, Bookheimer SY (2000) Averaging flat maps of hippocampal activity across subjects, 6th International Conference on Functional Mapping of the Human Brain, San Antonio, Texas, June 2000.
Zijdenbos AP, Dawant BM (1994) Brain segmentation and white matter lesion detection in MR images. Crit Rev Biomed Eng 22:401–465.[Medline]
![]() ). These new approaches were also designed to allow mapping of cortical asymmetries important in evaluating evidence for the asymmetrical progression of the disease. In this way, true differences in gray matter distribution and cortical metabolism can be distinguished from individual or hemispheric differences in cortical organization.
). These new approaches were also designed to allow mapping of cortical asymmetries important in evaluating evidence for the asymmetrical progression of the disease. In this way, true differences in gray matter distribution and cortical metabolism can be distinguished from individual or hemispheric differences in cortical organization. ![]() ). In addition, the patients had an acquired persistent decline involving at least three of the following domains: language, memory, visuospatial skills, cognition, emotion or personality (Cummings et al., 1980
). In addition, the patients had an acquired persistent decline involving at least three of the following domains: language, memory, visuospatial skills, cognition, emotion or personality (Cummings et al., 1980![]() ). Exclusion criteria for all subjects were the presence of a focal lesion on brain MRI, history of head trauma, past psychiatric history or an active medical problem.
). Exclusion criteria for all subjects were the presence of a focal lesion on brain MRI, history of head trauma, past psychiatric history or an active medical problem. ![]() ) was carefully matched across the patient cohort to reflect the mild AD population typically presenting initially to a clinic (Murphy et al., 1993
) was carefully matched across the patient cohort to reflect the mild AD population typically presenting initially to a clinic (Murphy et al., 1993![]() ). The 20 elderly control subjects were matched with the patients for age, sex, educational level and handedness (mean age 72.4 ± 1.3 years, 8 females/12 males, mean educational level 15.4 ± 0.5 years, all right-handed).
). The 20 elderly control subjects were matched with the patients for age, sex, educational level and handedness (mean age 72.4 ± 1.3 years, 8 females/12 males, mean educational level 15.4 ± 0.5 years, all right-handed). ![]() ). It forms the core of a growing disease-specific atlas of the brain in AD (Thompson et al., 2000a
). It forms the core of a growing disease-specific atlas of the brain in AD (Thompson et al., 2000a![]() ,b
,b![]() ,c
,c![]() ; Mega et al., 1997, 1998
; Mega et al., 1997, 1998![]() , 2000a
, 2000a![]() ,b
,b![]() ). Briefly, the average MRI brain template (Fig. 1c
). Briefly, the average MRI brain template (Fig. 1c![]() ) was constructed to have the average shape and size for a group of elderly subjects. Specialized approaches for anatomical averaging were used to generate an average MRI scan with well-resolved cortical features in their mean spatial locations. The resulting template reflects the average morphology of an elderly group of subjects and better reflects the anatomy of the subjects in this study than imaging templates based on young normals (Evans et al., 1994
) was constructed to have the average shape and size for a group of elderly subjects. Specialized approaches for anatomical averaging were used to generate an average MRI scan with well-resolved cortical features in their mean spatial locations. The resulting template reflects the average morphology of an elderly group of subjects and better reflects the anatomy of the subjects in this study than imaging templates based on young normals (Evans et al., 1994![]() ) (Fig. 1a
) (Fig. 1a![]() ) or post-mortem data (Talairach and Tournoux, 1988
) or post-mortem data (Talairach and Tournoux, 1988![]() ).
). ![]() ; Sled et al., 1998
; Sled et al., 1998![]() ) derived a 3D map of these low frequency signal fluctuations and divided by this scalar field to correct for any errors at the voxel level.
) derived a 3D map of these low frequency signal fluctuations and divided by this scalar field to correct for any errors at the voxel level. ![]() ). A nearest neighbor tissue classifier then assigned each image voxel to a particular tissue class (gray, white or CSF) or to a background class (representing extracerebral voxels in the image). The inter/intra-rater reliability of this protocol and its robustness to changes in image acquisition parameters have been described previously (Sowell et al., 1999a
). A nearest neighbor tissue classifier then assigned each image voxel to a particular tissue class (gray, white or CSF) or to a background class (representing extracerebral voxels in the image). The inter/intra-rater reliability of this protocol and its robustness to changes in image acquisition parameters have been described previously (Sowell et al., 1999a![]() ,b
,b![]() ). Gray matter maps were retained for subsequent analysis.
). Gray matter maps were retained for subsequent analysis. ![]() ). This algorithm successively deforms a spherical surface into the configuration of a given subject's cortex, resolving the gyral pattern. The ability of the 3D active surface extraction algorithm (MacDonald et al., 1993
). This algorithm successively deforms a spherical surface into the configuration of a given subject's cortex, resolving the gyral pattern. The ability of the 3D active surface extraction algorithm (MacDonald et al., 1993![]() , 1994
, 1994![]() , 1998) to extract high fidelity surface representations of the CSF/gray matter and gray/white matter interfaces in high resolution MR data has been extensively tested and validated in prior studies (Holmes et al., 1996
, 1998) to extract high fidelity surface representations of the CSF/gray matter and gray/white matter interfaces in high resolution MR data has been extensively tested and validated in prior studies (Holmes et al., 1996![]() ; Thompson et al., 1997
; Thompson et al., 1997![]() , 1998
, 1998![]() ; MacDonald, 1998
; MacDonald, 1998![]() ). The resulting cortical model consists of a mesh of discrete triangular elements that tile the surface. The intensity value at which the gray matter/CSF interface occurred was determined in 20–30 cortical regions and the threshold set to the mean. This threshold was independently validated in each case by comparing the automatically extracted surface boundaries with the manually determined surface of the gray matter–CSF boundary, identified at high magnification in the corresponding 3D MR volumes.
). The resulting cortical model consists of a mesh of discrete triangular elements that tile the surface. The intensity value at which the gray matter/CSF interface occurred was determined in 20–30 cortical regions and the threshold set to the mean. This threshold was independently validated in each case by comparing the automatically extracted surface boundaries with the manually determined surface of the gray matter–CSF boundary, identified at high magnification in the corresponding 3D MR volumes. ![]() ) were manually outlined on a highly magnified surface-rendered image of each cortex. Priority was given to biological features whose topological consistency has been demonstrated across normal populations (Ono et al., 1990
) were manually outlined on a highly magnified surface-rendered image of each cortex. Priority was given to biological features whose topological consistency has been demonstrated across normal populations (Ono et al., 1990![]() ; Le Goualher et al., 1996
; Le Goualher et al., 1996![]() ; MacDonald et al., 1997
; MacDonald et al., 1997![]() ). Detailed anatomical criteria were applied as previously set out (Steinmetz et al., 1989
). Detailed anatomical criteria were applied as previously set out (Steinmetz et al., 1989![]() , 1990
, 1990![]() ; Missir et al., 1989
; Missir et al., 1989![]() ; Leonard, 1996
; Leonard, 1996![]() ; Thompson and Toga, 1997
; Thompson and Toga, 1997![]() ; Thompson et al., 1997
; Thompson et al., 1997![]() ; Kennedy et al., 1998
; Kennedy et al., 1998![]() ) and as in a sulcal atlas (Ono et al., 1990
) and as in a sulcal atlas (Ono et al., 1990![]() ). In both hemispheres 3D curves were drawn to represent the superior and inferior frontal, central, post-central, intraparietal, superior and inferior temporal, collateral, olfactory and occipito-temporal sulci, as well as the Sylvian fissures. Additional 3D curves were drawn to represent gyral limits at the interhemispheric margin1 (Thompson et al., 1997
). In both hemispheres 3D curves were drawn to represent the superior and inferior frontal, central, post-central, intraparietal, superior and inferior temporal, collateral, olfactory and occipito-temporal sulci, as well as the Sylvian fissures. Additional 3D curves were drawn to represent gyral limits at the interhemispheric margin1 (Thompson et al., 1997![]() ). Stereotaxic locations of contour points derived from the data volume were re-digitized to produce 36 uniformly parameterized cortical contours per brain, representing the primary gyral pattern of each subject (Thompson et al., 1997
). Stereotaxic locations of contour points derived from the data volume were re-digitized to produce 36 uniformly parameterized cortical contours per brain, representing the primary gyral pattern of each subject (Thompson et al., 1997![]() ; Thompson and Toga, 1998
; Thompson and Toga, 1998![]() ).
). ![]() ), consistent with the suggestion from metabolic studies (Loewenstein et al., 1989
), consistent with the suggestion from metabolic studies (Loewenstein et al., 1989![]() ) that the left hemisphere is, on average, more severely affected at this stage of the disease. The occipital cortices were comparatively spared bilaterally, as were the sensorimotor cortices (0–5% loss, P > 0.05). There was also severe gray matter loss (20–30%, P < 0.001–0.0001) in the middle frontal gyrus, in the vicinity of areas 9 and 46 (Rajkowska and Goldman-Rakic, 1995
) that the left hemisphere is, on average, more severely affected at this stage of the disease. The occipital cortices were comparatively spared bilaterally, as were the sensorimotor cortices (0–5% loss, P > 0.05). There was also severe gray matter loss (20–30%, P < 0.001–0.0001) in the middle frontal gyrus, in the vicinity of areas 9 and 46 (Rajkowska and Goldman-Rakic, 1995![]() ). We further investigated whether the regions of more significant gray matter loss reflected a correspondingly greater average reduction in the local gray matter index (Fig. 7
). We further investigated whether the regions of more significant gray matter loss reflected a correspondingly greater average reduction in the local gray matter index (Fig. 7![]() ). This was important, as a greater significance value can result either from (i) a genuinely greater percent reduction in the mean gray matter in AD or (ii) a local reduction in the variance of the gray matter index across the group, which translates into a greater detection sensitivity. Interestingly, a map of the percentage reduction in average gray matter (Fig. 7
). This was important, as a greater significance value can result either from (i) a genuinely greater percent reduction in the mean gray matter in AD or (ii) a local reduction in the variance of the gray matter index across the group, which translates into a greater detection sensitivity. Interestingly, a map of the percentage reduction in average gray matter (Fig. 7![]() ) followed approximately the same anatomical pattern, suggesting that there is indeed a hierarchy in the severity of gray matter loss at this stage of the disease, rather than a fluctuation in the local power of the statistical model to detect it. Again, the temporal and temporo-parietal cortex exhibited severe (10–30%) reductions in gray matter. This contrasted with a comparative sparing of the superior margins of the central and post-central gyri and occipital poles (0–5% loss). Although diffuse gray matter loss is likely to occur across the majority of the cortex, it is interesting that the superior central and post-central gyri and occipital poles show very little reduction in gray matter when adjacent posterior temporal cortex and the parietal operculum are severely affected, in both the percentage loss and statistical anatomical maps.
) followed approximately the same anatomical pattern, suggesting that there is indeed a hierarchy in the severity of gray matter loss at this stage of the disease, rather than a fluctuation in the local power of the statistical model to detect it. Again, the temporal and temporo-parietal cortex exhibited severe (10–30%) reductions in gray matter. This contrasted with a comparative sparing of the superior margins of the central and post-central gyri and occipital poles (0–5% loss). Although diffuse gray matter loss is likely to occur across the majority of the cortex, it is interesting that the superior central and post-central gyri and occipital poles show very little reduction in gray matter when adjacent posterior temporal cortex and the parietal operculum are severely affected, in both the percentage loss and statistical anatomical maps. ![]() ). Gyral patterns did not vary equally in all directions and the statistical distribution that describes the location of a cortical region in space was elongated in a particular direction, which also varied locally across the cortex. To visualize this, cortical variations were modeled as vector field displacements of an average cortical model and ellipsoids of constant probability density were computed for positions of cortical regions (relative to the average cortex). Figure 9c
). Gyral patterns did not vary equally in all directions and the statistical distribution that describes the location of a cortical region in space was elongated in a particular direction, which also varied locally across the cortex. To visualize this, cortical variations were modeled as vector field displacements of an average cortical model and ellipsoids of constant probability density were computed for positions of cortical regions (relative to the average cortex). Figure 9c![]() shows the shape of a 3D Gaussian distribution fitted at each point on the average normal cortex, reflecting the cross-subject variation of points from equivalent gyral regions. The shape of this distribution at each cortical point is described by the covariance tensor of the 3D distribution. Its value determines a set of nested ellipsoids that represent confidence limits for the locations of corresponding anatomical points in stereotaxic space (Thompson et al., 1997
shows the shape of a 3D Gaussian distribution fitted at each point on the average normal cortex, reflecting the cross-subject variation of points from equivalent gyral regions. The shape of this distribution at each cortical point is described by the covariance tensor of the 3D distribution. Its value determines a set of nested ellipsoids that represent confidence limits for the locations of corresponding anatomical points in stereotaxic space (Thompson et al., 1997![]() ; Cao and Worsley, 2000
; Cao and Worsley, 2000![]() ). These ellipsoids (Fig. 9c
). These ellipsoids (Fig. 9c![]() ) are colored by the determinant of the covariance tensor, for which larger values (pink) represent greater 3D variability and small values (blue) represent regions whose morphology is highly conserved across subjects.
) are colored by the determinant of the covariance tensor, for which larger values (pink) represent greater 3D variability and small values (blue) represent regions whose morphology is highly conserved across subjects. ![]() ). In several cortical regions the principal directions of variability (along which the glyphs are elongated in Fig. 9
). In several cortical regions the principal directions of variability (along which the glyphs are elongated in Fig. 9![]() ) were approximately orthogonal to the primary gyral pattern. This directional trend was similar in some respects to the torquing, or petalia, which causes cortical regions in the right hemisphere to be situated slightly anterior to their counterparts on the left (Galaburda and Geschwind, 1981
) were approximately orthogonal to the primary gyral pattern. This directional trend was similar in some respects to the torquing, or petalia, which causes cortical regions in the right hemisphere to be situated slightly anterior to their counterparts on the left (Galaburda and Geschwind, 1981![]() ; Bilder et al., 1994
; Bilder et al., 1994![]() ). The region of highly anisotropic variability was strongly localized to the temporo-parietal cortex and did not extend anteriorly into the post-central and central gyri. A marked anatomical division occurred at the post-central gyrus, where variability was reduced and was spatially more isotropic. The component of variability normal to the average cortex was greatest at the temporal poles, where gyral patterns are relatively stable and variations in temporal lobe size may dominate. Importantly, this directional cortical variability is controlled by surface matching within the continuum-mechanical atlas, thereby allowing accurate maps of disease-related gray matter loss to be constructed (Figs 5,7
). The region of highly anisotropic variability was strongly localized to the temporo-parietal cortex and did not extend anteriorly into the post-central and central gyri. A marked anatomical division occurred at the post-central gyrus, where variability was reduced and was spatially more isotropic. The component of variability normal to the average cortex was greatest at the temporal poles, where gyral patterns are relatively stable and variations in temporal lobe size may dominate. Importantly, this directional cortical variability is controlled by surface matching within the continuum-mechanical atlas, thereby allowing accurate maps of disease-related gray matter loss to be constructed (Figs 5,7![]()
![]() ).
). ![]() illustrates the group average patterns of cortical asymmetry, highlighting regional trends. In a previous study we found Sylvian fissure asymmetry to be significantly greater in AD (P < 0.05) than in controls matched for age, gender and handedness (Thompson et al., 1998
illustrates the group average patterns of cortical asymmetry, highlighting regional trends. In a previous study we found Sylvian fissure asymmetry to be significantly greater in AD (P < 0.05) than in controls matched for age, gender and handedness (Thompson et al., 1998![]() ). Although these asymmetries are not apparent in every individual, a localized region can be clearly defined in which major asymmetrical trends are present (Fig. 10
). Although these asymmetries are not apparent in every individual, a localized region can be clearly defined in which major asymmetrical trends are present (Fig. 10![]() ). Severe asymmetry exhibited by the posterior Sylvian fissure (up to 10 mm) contrasted with negligible asymmetry in the frontal, parietal and occipital cortex (1–2 mm). The group average anatomy (Fig. 10
). Severe asymmetry exhibited by the posterior Sylvian fissure (up to 10 mm) contrasted with negligible asymmetry in the frontal, parietal and occipital cortex (1–2 mm). The group average anatomy (Fig. 10![]() ) shows the average Sylvian fissure terminating more posteriorly (P < 0.0002) and oriented more horizontally on the left than the right, corroborating postmortem measurements of the planum temporale (Geschwind and Levitsky, 1968
) shows the average Sylvian fissure terminating more posteriorly (P < 0.0002) and oriented more horizontally on the left than the right, corroborating postmortem measurements of the planum temporale (Geschwind and Levitsky, 1968![]() , Witelson and Kigar, 1992
, Witelson and Kigar, 1992![]() ; Galaburda, 1995
; Galaburda, 1995![]() ). The average right Sylvian fissure also shows an upward turn at its posterior limit (Fig. 10
). The average right Sylvian fissure also shows an upward turn at its posterior limit (Fig. 10![]() ) and is anterior to the posterior limit on the left (Thompson et al., 1998
) and is anterior to the posterior limit on the left (Thompson et al., 1998![]() ).
). ![]() ) of predominant left hemisphere metabolic dysfunction in mild to moderate AD, when cerebral glucose utilization is measured by positron emission tomography (PET). Structural, perfusion and metabolic studies suggest that the left hemisphere may be more susceptible to neuronal loss, instead of the alternative explanation that equivalent neuronal loss may result in greater functional deficits on one side, due to asymmetrical cortical organization. Greatest gray matter loss in the temporo-parietal cortex may underlie the prominent temporal-parietal hypometabolism that is consistently found at this stage of AD, often asymmetrically (Friedland and Luxenberg, 1988
) of predominant left hemisphere metabolic dysfunction in mild to moderate AD, when cerebral glucose utilization is measured by positron emission tomography (PET). Structural, perfusion and metabolic studies suggest that the left hemisphere may be more susceptible to neuronal loss, instead of the alternative explanation that equivalent neuronal loss may result in greater functional deficits on one side, due to asymmetrical cortical organization. Greatest gray matter loss in the temporo-parietal cortex may underlie the prominent temporal-parietal hypometabolism that is consistently found at this stage of AD, often asymmetrically (Friedland and Luxenberg, 1988![]() ; Johnson et al., 1998
; Johnson et al., 1998![]() ). Although the focus of this study was to determine patterns of gray matter loss in vivo, immunocytochemical studies have reported between 11 and 50% synaptic loss in the superior temporal and inferior parietal cortices, with a comparative sparing of occipital cortices (cf. Figs 5,7
). Although the focus of this study was to determine patterns of gray matter loss in vivo, immunocytochemical studies have reported between 11 and 50% synaptic loss in the superior temporal and inferior parietal cortices, with a comparative sparing of occipital cortices (cf. Figs 5,7![]()
![]() ). Relatively greater atrophy is often reported in the temporal lobe relative to overall cerebral volume (Murphy et al., 1993
). Relatively greater atrophy is often reported in the temporal lobe relative to overall cerebral volume (Murphy et al., 1993![]() ). The early progression of AD pathology into the parietal and frontal association cortices suggests a degeneration of synaptically linked cortical pathways, and this pattern correlates with symptoms of memory impairment, aphasias, apraxias, personality changes and spatial deficits (Roberts et al., 1993
). The early progression of AD pathology into the parietal and frontal association cortices suggests a degeneration of synaptically linked cortical pathways, and this pattern correlates with symptoms of memory impairment, aphasias, apraxias, personality changes and spatial deficits (Roberts et al., 1993![]() ). Interestingly, gray matter loss at autopsy is predominantly cortical in Alzheimer's patients under 80 years of age (Hubbard and Anderson, 1981
). Interestingly, gray matter loss at autopsy is predominantly cortical in Alzheimer's patients under 80 years of age (Hubbard and Anderson, 1981![]() ), when volumes of subcortical nuclei are not significantly different between patients and controls (De La Monte, 1989
), when volumes of subcortical nuclei are not significantly different between patients and controls (De La Monte, 1989![]() ). Nonetheless, atrophy of the amygdala and basal nuclei (Cuénod et al., 1993
). Nonetheless, atrophy of the amygdala and basal nuclei (Cuénod et al., 1993![]() ) may ultimately be followed by alterations in thalamic nuclei (Jernigan et al., 1991
) may ultimately be followed by alterations in thalamic nuclei (Jernigan et al., 1991![]() ), induced perhaps by degeneration of their cortical projection areas.
), induced perhaps by degeneration of their cortical projection areas. ![]() ). Gray matter loss within a gyrus may be a multifocal process (as, for example, the discrete lesions in vascular dementia) or may occur rather uniformly within individual gyri. Clearly, some features occur at small spatial scales in both the statistical (P value) maps and the average loss maps. This multifocal effect does not appear to be attributable to sampling error in estimating the variance for the gray matter measure, as these variance values are spatially quite homogeneous. Structural and functional features with a spatial scale smaller than a gyrus may begin to be resolved if data from corresponding gyri are better aligned across subjects when averaging features from a population (Thompson et al., 2000a
). Gray matter loss within a gyrus may be a multifocal process (as, for example, the discrete lesions in vascular dementia) or may occur rather uniformly within individual gyri. Clearly, some features occur at small spatial scales in both the statistical (P value) maps and the average loss maps. This multifocal effect does not appear to be attributable to sampling error in estimating the variance for the gray matter measure, as these variance values are spatially quite homogeneous. Structural and functional features with a spatial scale smaller than a gyrus may begin to be resolved if data from corresponding gyri are better aligned across subjects when averaging features from a population (Thompson et al., 2000a![]() ; Zeineh et al., 2000
; Zeineh et al., 2000![]() ). Conversely, gyral features may be blurred out (cf. Fig. 3
). Conversely, gyral features may be blurred out (cf. Fig. 3![]() ) when these correspondences are not taken into account (Evans et al., 1994
) when these correspondences are not taken into account (Evans et al., 1994![]() ). We did not hypothesize this multifocal effect in advance, so we did not test for its significance specifically. Longitudinal studies may allow us to better understand the scale and consistency of these localized changes over time and may reveal whether gray matter loss is an inherently diffuse or multifocal process within individual cortical gyri.
). We did not hypothesize this multifocal effect in advance, so we did not test for its significance specifically. Longitudinal studies may allow us to better understand the scale and consistency of these localized changes over time and may reveal whether gray matter loss is an inherently diffuse or multifocal process within individual cortical gyri. ![]() ), as well as many cyto-architectonic regions (Rademacher et al., 1993
), as well as many cyto-architectonic regions (Rademacher et al., 1993![]() ), bear a consistent relationship to macroanatomical landmarks of the gyral pattern. The degree to which cortical pattern matching reduces architectonic and functional variation can be evaluated by quantifying residual variability of functional or cellular landmarks after normalizing gross anatomical features (Rajkowska and Goldman-Rakic, 1995
), bear a consistent relationship to macroanatomical landmarks of the gyral pattern. The degree to which cortical pattern matching reduces architectonic and functional variation can be evaluated by quantifying residual variability of functional or cellular landmarks after normalizing gross anatomical features (Rajkowska and Goldman-Rakic, 1995![]() ; Van Essen and Drury, 1997
; Van Essen and Drury, 1997![]() ; Fox et al., 1999
; Fox et al., 1999![]() ; Geyer et al., 2000
; Geyer et al., 2000![]() ). Differences in the topological layout of architectonic regions within the cortical sheet ultimately preclude the mapping of discrete cortical regions from one subject to another, so an important intermediate goal has been to identify and match a comprehensive network of sulcal and gyral elements which are consistent in their incidence and topology across subjects (Ono et al., 1990
). Differences in the topological layout of architectonic regions within the cortical sheet ultimately preclude the mapping of discrete cortical regions from one subject to another, so an important intermediate goal has been to identify and match a comprehensive network of sulcal and gyral elements which are consistent in their incidence and topology across subjects (Ono et al., 1990![]() ; Rademacher et al., 1993
; Rademacher et al., 1993![]() ; Thompson et al., 1996a
; Thompson et al., 1996a![]() , 1997
, 1997![]() ). While gyral matching substantially reduces the variability in cortical organization across subjects, in the future functional and architectonic landmarks may be definable in vivo that better guarantee matching of the cortical mantle from one subject to another in population studies (Dumoulin et al., 2000
). While gyral matching substantially reduces the variability in cortical organization across subjects, in the future functional and architectonic landmarks may be definable in vivo that better guarantee matching of the cortical mantle from one subject to another in population studies (Dumoulin et al., 2000![]() ).
). ![]() ). Clear differences in both AD cortical variation and gray matter distribution suggest the need for disease-specific brain atlases that better reflect the disease-related anatomy of patients and calibrate individual loss against statistical data from normative populations.
). Clear differences in both AD cortical variation and gray matter distribution suggest the need for disease-specific brain atlases that better reflect the disease-related anatomy of patients and calibrate individual loss against statistical data from normative populations. ![]() , the marked anatomical asymmetry in the posterior perisylvian cortex (Geschwind and Levitsky, 1968
, the marked anatomical asymmetry in the posterior perisylvian cortex (Geschwind and Levitsky, 1968![]() ) extends rostrally into the post-central cortex. The posterior bank of the post-central gyrus is thrust forward by 8–9 mm on the right compared with the left (Fig. 10
) extends rostrally into the post-central cortex. The posterior bank of the post-central gyrus is thrust forward by 8–9 mm on the right compared with the left (Fig. 10![]() ). This asymmetry extends caudally across the lateral convexity into the superior and inferior temporal cortex. As shown by averaging models of ventricular anatomy (Thompson et al., 2000d
). This asymmetry extends caudally across the lateral convexity into the superior and inferior temporal cortex. As shown by averaging models of ventricular anatomy (Thompson et al., 2000d![]() ), this asymmetrical trend penetrates subcortically into the occipital horns of the lateral ventricles, but not into adjacent parieto-occipital and calcarine cortex (Thompson et al., 1998
), this asymmetrical trend penetrates subcortically into the occipital horns of the lateral ventricles, but not into adjacent parieto-occipital and calcarine cortex (Thompson et al., 1998![]() ). In contrast with existing brain atlases based on a single brain hemisphere (Talairach and Tournoux, 1988
). In contrast with existing brain atlases based on a single brain hemisphere (Talairach and Tournoux, 1988![]() ), population-based atlases encode information on asymmetry and its group variation, so that departures from normal patterns in individuals or groups can be identified (Thompson et al., 1997
), population-based atlases encode information on asymmetry and its group variation, so that departures from normal patterns in individuals or groups can be identified (Thompson et al., 1997![]() ; Thirion et al., 1998
; Thirion et al., 1998![]() ; Thompson and Toga, 1998
; Thompson and Toga, 1998![]() ; Cao and Worsley, 2000
; Cao and Worsley, 2000![]() ). There is a substantial literature on Sylvian fissure cortical surface asymmetries (Eberstaller, 1884
). There is a substantial literature on Sylvian fissure cortical surface asymmetries (Eberstaller, 1884![]() ; Cunningham, 1892
; Cunningham, 1892![]() ; Geschwind and Levitsky, 1968
; Geschwind and Levitsky, 1968![]() ; Davidson and Hugdahl, 1994
; Davidson and Hugdahl, 1994![]() ) and their relation to functional lateralization (Strauss et al., 1983
) and their relation to functional lateralization (Strauss et al., 1983![]() ), handedness (Witelson and Kigar, 1992
), handedness (Witelson and Kigar, 1992![]() ), language function (Davidson and Hugdahl, 1994
), language function (Davidson and Hugdahl, 1994![]() ), asymmetries of associated cytoarchitectonic fields (Galaburda and Geschwind, 1981
), asymmetries of associated cytoarchitectonic fields (Galaburda and Geschwind, 1981![]() ) and their thalamic projection areas (Eidelberg and Galaburda, 1982
) and their thalamic projection areas (Eidelberg and Galaburda, 1982![]() ), However, no prior reports have mapped the asymmetry profile across the cortex in three dimensions. These localized patterns of asymmetry in cortical morphology clearly have multiple determinants. We previously found Sylvian fissure asymmetry to be significantly greater in AD patients than in controls matched for age, gender, educational level and handedness (P < 0.05) (Thompson et al., 1998
), However, no prior reports have mapped the asymmetry profile across the cortex in three dimensions. These localized patterns of asymmetry in cortical morphology clearly have multiple determinants. We previously found Sylvian fissure asymmetry to be significantly greater in AD patients than in controls matched for age, gender, educational level and handedness (P < 0.05) (Thompson et al., 1998![]() ), suggesting that AD pathology asymmetrically disrupts the anatomy of the temporo-parietal cortex. The improved ability to localize asymmetries of cortical organization or tissue loss in a group atlas presents opportunities to analyze diseases with asymmetrical progression, including different stages of AD, and to map hypothesized alterations in cortical and hippocampal asymmetry in disease states such as schizophrenia (Falkai et al., 1992
), suggesting that AD pathology asymmetrically disrupts the anatomy of the temporo-parietal cortex. The improved ability to localize asymmetries of cortical organization or tissue loss in a group atlas presents opportunities to analyze diseases with asymmetrical progression, including different stages of AD, and to map hypothesized alterations in cortical and hippocampal asymmetry in disease states such as schizophrenia (Falkai et al., 1992![]() ; Kikinis et al., 1994
; Kikinis et al., 1994![]() ; Kulynych et al., 1996
; Kulynych et al., 1996![]() ; Csernansky et al., 1998
; Csernansky et al., 1998![]() ).
). ![]() ) and for individual structures such as the corpus callosum (Gee et al., 1995
) and for individual structures such as the corpus callosum (Gee et al., 1995![]() ; Davatzikos, 1996
; Davatzikos, 1996![]() ), central sulcus (Manceaux-Demiau et al., 1998
), central sulcus (Manceaux-Demiau et al., 1998![]() ), cingulate and paracingulate sulci (Paus et al., 1996
), cingulate and paracingulate sulci (Paus et al., 1996![]() ), hippocampus (Haller et al., 1997
), hippocampus (Haller et al., 1997![]() ; Csernansky et al., 1998
; Csernansky et al., 1998![]() ; Joshi et al., 1998
; Joshi et al., 1998![]() ) and for transformed representations of the human and macaque cortex (Drury and Van Essen, 1997
) and for transformed representations of the human and macaque cortex (Drury and Van Essen, 1997![]() ; Grenander and Miller, 1998
; Grenander and Miller, 1998![]() ; Fischl et al., 1999
; Fischl et al., 1999![]() ). The resulting averages provide templates in which multimodality brain maps can be integrated (Mazziotta et al., 1995
). The resulting averages provide templates in which multimodality brain maps can be integrated (Mazziotta et al., 1995![]() ; Toga and Thompson, 1998
; Toga and Thompson, 1998![]() ). The probabil-istic information they contain can also guide Bayesian approaches for automatically identifying anatomical structures (Gee et al., 1995
). The probabil-istic information they contain can also guide Bayesian approaches for automatically identifying anatomical structures (Gee et al., 1995![]() ; Mangin et al., 1995
; Mangin et al., 1995![]() ; Royackkers et al., 1996
; Royackkers et al., 1996![]() ; Pitiot et al., 2000
; Pitiot et al., 2000![]() ). Finally, these probabilistic atlases can constrain the search space for activations in functional imaging experiments (Dinov et al., 2000
). Finally, these probabilistic atlases can constrain the search space for activations in functional imaging experiments (Dinov et al., 2000![]() ).
). ![]() ). Higher rates of atrophy and tissue loss have been estimated for specific structures, including the hippocampus (Kaye et al., 1997
). Higher rates of atrophy and tissue loss have been estimated for specific structures, including the hippocampus (Kaye et al., 1997![]() ; Jack et al., 1998
; Jack et al., 1998![]() ; Laakso et al., 2000
; Laakso et al., 2000![]() ). Four-dimensional maps of degenerative rates may also be derived by computing a deformation field that elastically transforms a subject's anatomy from its earlier configuration to its shape in a later scan (Fox et al., 1996
). Four-dimensional maps of degenerative rates may also be derived by computing a deformation field that elastically transforms a subject's anatomy from its earlier configuration to its shape in a later scan (Fox et al., 1996![]() , 2000
, 2000![]() ; Thompson et al., 2000b
; Thompson et al., 2000b![]() ,d
,d![]() ). We are currently extending the mapping approach described here to store detailed population-based maps of degenerative rates across time and explore linkages between these maps and cognitive variables (Thompson et al., 2000d
). We are currently extending the mapping approach described here to store detailed population-based maps of degenerative rates across time and explore linkages between these maps and cognitive variables (Thompson et al., 2000d![]() ), as well as therapeutic and genetic factors [e.g. ApoE genotype (Small et al., 2000)].
), as well as therapeutic and genetic factors [e.g. ApoE genotype (Small et al., 2000)]. ![]() ), statistical maps of gray matter loss at multiple time points will ultimately provide a dynamic frame-work to help understand the progression of the disease and to gauge therapeutic, disease-modifying response in an individual or clinically defined group.
), statistical maps of gray matter loss at multiple time points will ultimately provide a dynamic frame-work to help understand the progression of the disease and to gauge therapeutic, disease-modifying response in an individual or clinically defined group. 
![]() ) and is summarized here for completeness. Since each cortical model is obtained by deforming a spherical surface into the shape of the cortex, gyral features can be mapped back onto a sphere and, subsequently, to a plane (Fig. 2
) and is summarized here for completeness. Since each cortical model is obtained by deforming a spherical surface into the shape of the cortex, gyral features can be mapped back onto a sphere and, subsequently, to a plane (Fig. 2![]() ). This simplifies computation of anatomical correspondences. Anatomical correspondences can therefore be computed by defining a flow field in the flat, 2D parameter space that matches gyral features from one subject to another (Figs 2, 3
). This simplifies computation of anatomical correspondences. Anatomical correspondences can therefore be computed by defining a flow field in the flat, 2D parameter space that matches gyral features from one subject to another (Figs 2, 3![]()
![]() ) (Davatzikos et al., 1996
) (Davatzikos et al., 1996![]() ; Thompson et al., 1996a
; Thompson et al., 1996a![]() , 1997
, 1997![]() , 2000d
, 2000d![]() ; Fischl et al., 1999
; Fischl et al., 1999![]() ). The flow is given by the solution to a curve-driven warp in the flat parametric space of the cortex (Thompson et al., 1996, 1998
). The flow is given by the solution to a curve-driven warp in the flat parametric space of the cortex (Thompson et al., 1996, 1998![]() , 2000). The flow behavior is modeled using equations derived from continuum mechanics and these equations are governed by the Cauchy–Navier differential operator L = µ
, 2000). The flow behavior is modeled using equations derived from continuum mechanics and these equations are governed by the Cauchy–Navier differential operator L = µ 2 + (
2 + (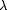 + µ)
+ µ) (
( T•) (Davatzikos et al., 1996
T•) (Davatzikos et al., 1996![]() ; Thompson et al., 1996, 1998
; Thompson et al., 1996, 1998![]() , 2000d
, 2000d![]() ; Grenander and Miller, 1998
; Grenander and Miller, 1998![]() ).
).  = [0,2
= [0,2 ) x [0,
) x [0, ), a system of simultaneous partial differential equations can be written for the flow field u (r):
), a system of simultaneous partial differential equations can be written for the flow field u (r): 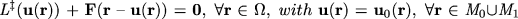
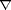 2 + (
2 + (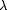 + µ)
+ µ)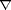 (
(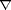 T•) with body force F (Thompson et al., 1996, 1998
T•) with body force F (Thompson et al., 1996, 1998![]() , 2000; Grenander and Miller, 1998
, 2000; Grenander and Miller, 1998![]() ). In solving this governing equation matching sulcal networks across subjects, dependencies between the metric tensors of the surface parameterizations and the matching field are eliminated with an approach known as covariant regularization, which uses generalized coordinates and correction terms known as Christoffel symbols (Thompson and Toga, 2000a,d). Because of the intrinsic curvature of the cortex, this means that the ‘covariant form’ L
). In solving this governing equation matching sulcal networks across subjects, dependencies between the metric tensors of the surface parameterizations and the matching field are eliminated with an approach known as covariant regularization, which uses generalized coordinates and correction terms known as Christoffel symbols (Thompson and Toga, 2000a,d). Because of the intrinsic curvature of the cortex, this means that the ‘covariant form’ L of the differential operator L is used when solving these equations (Thompson and Toga, 1998
of the differential operator L is used when solving these equations (Thompson and Toga, 1998![]() ; Thompson et al., 2000d
; Thompson et al., 2000d![]() ). This adjustment also makes sure that cortical surfaces are matched in a way that is actually independent of the way the surfaces are flattened; in other words, the matching procedure is parameterization-invariant. In the partial differential equations (2)
). This adjustment also makes sure that cortical surfaces are matched in a way that is actually independent of the way the surfaces are flattened; in other words, the matching procedure is parameterization-invariant. In the partial differential equations (2)![]() we replace L by the covariant differential operator L
we replace L by the covariant differential operator L . In L
. In L all L value partial derivatives are replaced by covariant derivatives. These covariant derivatives are defined with respect to the metric tensor of the surface domain where calculations are performed. The covariant derivative of a (contravariant) vector field, ui(x), is defined as ui,k =
all L value partial derivatives are replaced by covariant derivatives. These covariant derivatives are defined with respect to the metric tensor of the surface domain where calculations are performed. The covariant derivative of a (contravariant) vector field, ui(x), is defined as ui,k =  uj/
uj/ xk +
xk +  jik ui, where the Christoffel symbols of the second kind,
jik ui, where the Christoffel symbols of the second kind, 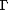 jik, are computed from derivatives of the metric tensor components gjk(x):
jik, are computed from derivatives of the metric tensor components gjk(x): 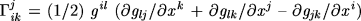
![]() ) representing 3D cortical point locations in an individual subject is convected along with the flow that drives the sulcal pattern into the average configuration for the group (Fig. 2f
) representing 3D cortical point locations in an individual subject is convected along with the flow that drives the sulcal pattern into the average configuration for the group (Fig. 2f![]() ). Once this is done in all subjects at a particular location in the flat map (Fig. 2f
). Once this is done in all subjects at a particular location in the flat map (Fig. 2f![]() ), points on each individual's cortex are recovered that have the same relative location to the primary folding pattern in all subjects. Averaging of these corresponding points results in a crisp average cortex (Fig. 3
), points on each individual's cortex are recovered that have the same relative location to the primary folding pattern in all subjects. Averaging of these corresponding points results in a crisp average cortex (Fig. 3![]() , bottom row). The corresponding 3D displacement is recovered between the cortical models. This displacement matches a large network of sulcal features and thus is a valid encoding of gyral pattern differences.
, bottom row). The corresponding 3D displacement is recovered between the cortical models. This displacement matches a large network of sulcal features and thus is a valid encoding of gyral pattern differences. ![]() ). This corrected P value depends on the smoothness tensor of the residuals of the statistical model, which can also be estimated from the surface data, using an approach known as statistical flattening (Worsley et al., 1999
). This corrected P value depends on the smoothness tensor of the residuals of the statistical model, which can also be estimated from the surface data, using an approach known as statistical flattening (Worsley et al., 1999![]() ; Thompson et al., 2000d
; Thompson et al., 2000d![]() ).
). ![]()
![]() Abstract of this Article
Abstract of this Article
![]() Reprint (PDF) Version of this Article
Reprint (PDF) Version of this Article
![]() Similar articles found in:
Similar articles found in:
![]()
![]() Cereb. Cortex Online
Cereb. Cortex Online
![]() Search Medline for articles by:
Search Medline for articles by:![]()
![]() Thompson, P. M.
||
Toga, A. W.
Thompson, P. M.
||
Toga, A. W.
![]() Alert me when:
Alert me when:
![]()
![]() new articles cite this article
new articles cite this article